Frost circles/Polygon method.
This is a very useful mnemonic device for quickly setting down molecular diagrams for cyclic systems.
Rules for drawing frost circles –
- A circle of diameter 4β (radius 2β) is drawn.
- A regular polygon (all sides are equal) of n sides is inscribed in this circle, with one corner/vertex at the lowest point. n ⇒ # of atoms in the cyclic system.
- The points at which the corners of the polugon touch the circle, define the energy levels.
- The horizontal axis (diameter) corresponds to ‘α‘.
- Below the energy level α all points represent the bonding MOs and above it are antibonding MOs. The points on the diameter are non- bonding.
Let us draw some diagrams to understand this concept better.
I] Consider a three membered ring (n=3) .It has two π electrons.
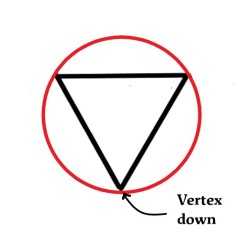
Now we inscribe this triangle in a circle, with one vertex down-
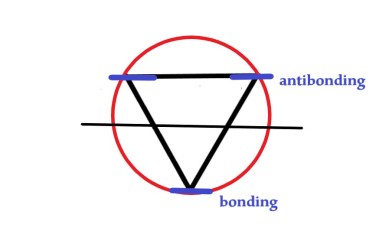
We draw a horizontal line dividing the circle into two halves. The points where the vertices of the triangle meet the circle are the energy levels. The energy levels above the diameter are antibonding and below it are bonding.
The two electrons will pair up in the lowest energy level as follows –
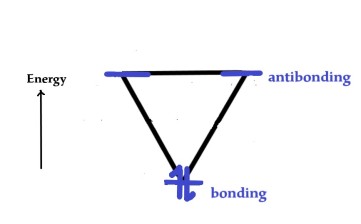
As both the electrons are in the bonding MO, the cation is stable and aromatic.
II] Consider, 1,3-cyclobutadiene (n=4).
 How should the frost diagram look like?
How should the frost diagram look like?
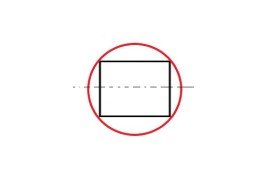
Can the diagram look like this? The answer is NO. In the adjacent figure, two vertices instead of one are at the base. So, the correct diagram should look like –
 Total energy = 2(α+2β)+2α = 4α+4β.
Total energy = 2(α+2β)+2α = 4α+4β.
III] Benzene (n= 6) has six π electrons. It’s frost diagram will look like –

Total energy = 2(α+2β) + 4(α+β) = 6α+4β
Cyclobutadiene has non-bonding MOs. Such non-bonding MOs are NOT present in aromatic systems like benzene.
In the next post we will compare benzene with cyclobutadiene and see how HMO theory explains their stabilities. Till then ,
Be a perpetual student of life and keep learning….
Good Day !
This is very helpful thank you.
LikeLike
I am glad it was helpful 🙂
LikeLike
THIS IS VERY WONDERFUL DETAILS
LikeLike