With this post, we start a long discourse on a fundamental topic at the core of Chemistry and the understanding of the whole universe – THE ATOMIC STRUCTURE. We know, that everything in this universe is made up of matter. But what is this matter made up of? Let us begin our journey into the sub-atomic realm. Let us start learning the secrets of this universe. Let us raise the curtain and study various phenomena previously enshrouded in mystery.
It all started in the 5th century BC, when a Greek philosopher named Leucippus, stated that everything is composed of imperishable, indivisible elements called ‘atoms’. (In Greek, ‘atomos‘ means indivisible/uncuttable). So, the word ‘ATOM’ originated in the Greek language. His pupil, Democritus later adopted Leucippus’s theory.

According to Democritus –
♦ The physical world consists of VOID (vacuum) and BEING (infinity of atoms).
♦ All matter consists of atoms which are indivisible and indestructible.

As mentioned in post 2, the atomic theory was proposed in India too. by Acharya Kannada (originally known by the name Kashyap). He was regarded as ‘The father of atomic theory’. He formulated the theory of tiny, invisible particles – ‘Anu’ (comparable to atoms), an indivisible entity that cannot be sensed through any human organ.
Let us fast forward to a few years later, from ancient civilisation to the modern world, and learn about the discoveries made in the 18th century.
In 1803, an atomic theory was proposed by John Dalton. John Dalton was a Fellow of the Royal Society (FRS). He made significant contributions to many branches of Chemistry, Physics and meteorology. He was a polymath who worked on gas laws, reflection and refraction of light, human vision, etc. He suffered from red-green colour blindness. This led him to work on the human eye and colour blindness.

The Dalton’s theory states –
♦ All matter is composed of atoms. Atoms are the smallest indivisible part of matter.
♦ Atoms cannot be created or destroyed.
♦ Atoms of different elements have different chemical properties and weights.
♦ Chemical reactions occur when atoms are rearranged, combined or separated.
♦ Atoms of different elements combine in simple whole-number ratios to form compounds.
Dalton rearranged the elements (known at that time) according to the ascending order of chemical mass and coined symbols for them. He was the first chemist to get a sense of stoichiometry in chemical reactions. He established a correlation between the difference in atomic structure and the chemical behaviour of elements! He also published a table of relative atomic weights. John Dalton suggested that atoms of elements have a characteristic mass. Thus, the formation of a table for elements began with John Dalton!


In 1897, Sir J.J. Thompson was studying the cathode rays. He established that cathode rays are negatively charged particles.
He applied a high voltage between two electrodes. These electrodes were fitted in a glass tube with vacuum inside. He observed that the rays were traveling from cathode to anode. As these rays were coming out of the cathode, he called them the ‘Cathode Rays‘.
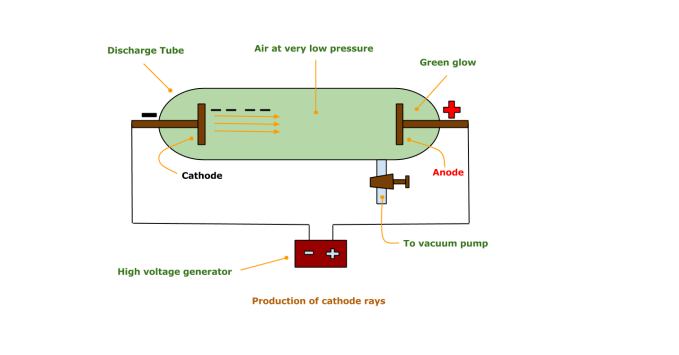
Eventually, he applied another electric field in the path of these cathode rays. The rays were deflected to the positive electric plate of the applied electric field. Thus, he concluded that these rays consisted of negatively charged particles. He thus discovered the first subatomic particle – THE ELECTRON ! (He called them negatively charged corpuscles).
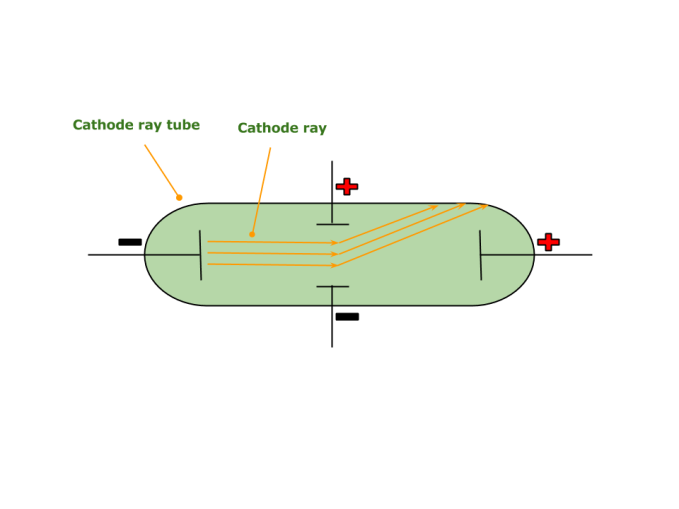
View the Cathode ray experiment here.
He used to say a toast at dinnertime,-
“To the electron! May it never be of any use to anybody!”
He not only discovered the electron but also characterized it. He found out the charge-to-mass ratio (e/m) for an electron. He won the NOBLE PRIZE for this discovery in 1906. Sir Thompson never coined the term electron, he called the negatively charged particles ‘corpuscles‘.
Irish electrochemist J. Stoney coined the word ‘Electron. He was an FRS too. This name came from the Greek word ‘Electra‘ which means amber. Amber is a kind of resin that comes out of trees or fossils. If amber is rubbed, a static charge is built on it. Thus, he thought of this word while naming the charged particle.
He also discovered that the electron was about 2000 times lighter than the hydrogen atom! Though he could get a sense of the electron’s mass, he could not exactly determine the charge of the electron.
One can measure the charge , mass and spin of an electron.One can study the effect of electrons on other particles.However, THE ELECTRON ITSELF HAS NEVER ACTUALLY BEEN OBSERVED!! THE ELECTRON IS SMALLER THAN ANYTHING EVER MEASURED !
After the discovery of electron, in 1904, J.J. Thompson proposed another atomic model – THE PLUM PUDDING MODEL.
THE PLUM PUDDING MODEL
Plum pudding is a common English dessert served especially during Christmas time.

Sir J.J. Thompson proposed that the structure of the atom is like a plum pudding – the negative corpuscles (electrons) were like plums in a positively charged pudding. The electrons were spread throughout the atom. According to this theory, the electrons were distributed in a spherical cloud of positive charge as follows –
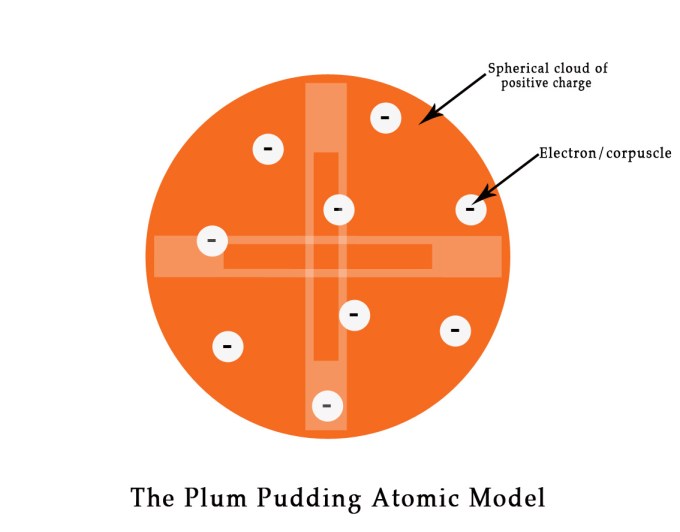
However, this model had shortcomings. It could not explain the spectral lines that were observed for some elements. As the proposed model did not corroborate spectroscopic data, this theory could not be considered foolproof. Although, the theory did not correctly explain the structure of an atom, it paved the way for one of J.J.Thompson’s student’s research in the Cavendish Lab at the University of Cambridge. This student of J.J.Thompson did some superlative work in the field of nuclear physics and came to be known as ‘The Father of Nuclear Physics‘ ! Who was this great scientist? What were his achievements? What progress did the 19th century witness with respect to the atomic structure studies? We shall study all of this is my coming posts.Till then ,
Be a perpetual student of life and keep learning ..
Good Day!
References and Further Reading –
- https://en.wikipedia.org/wiki/Atomic_theory
- https://the-history-of-the-atom.wikispaces.com/Democritus
- MIT 3.091SC Introduction to Solid State Chemistry, Fall 2010 Lecture by Professor Donald Sadoway.
- The Inexplicable Universe documentary.
Image sources –
1.http://edtech2.boisestate.edu/lindabennett1/502/democritus.html
2.http://www.johndalton.org/john-daltons-early-years/
3.http://firstip.org/legendary-scientists/maharshi-kanada-the-propunder-of-atomic-theory600-bce
4.https://en.wikipedia.org/wiki/J._J._Thomson
5.http://www.foodnetwork.ca/recipe/grand-plum-pudding/12722/
6.http://www.chemistryland.com/CHM130W/03-BuildingBlocks/Chaos/ChaosQuiz.htm
7.http://padakshep.org/otp/subjects/chemistry/physical-chemistry/discovery-of-electrons-protons-and-neutrons/