Scrupulously proved to be true in theory, any scientific thesis cannot be considered absolute until it is proved by experimental data. As a theoretical physicist, Louis Victor De Broglie successfully proposed a plausible idea that corroborated Niels Bohr’s quantum condition. However, without valid proof in the form of experimental data, the theory could not be unanimously accepted.
The experimental evidence for De Broglie’s theory came in 1927 at Bell Labs in New Jersey. An experiment proved the wave nature of particles to be true. However, before we learn the experiment, let us get acquainted with a few important wave concepts.
Wave Concepts
A] Interference –
Two waves travelling in the same medium tend to superpose, i.e. get one above the other. The resultant wave formed due to this superposition could have an amplitude greater than or less than the two parent waves. This phenomenon is called as interference of waves.
Check out this animation –
File:Waventerference.gif (The green and blue are parent waves, and the red wave is the resultant wave.)
There are two kinds of interference –
1) Constructive Interference and
2) Destructive Interference.
Consider two waves travelling in the same medium – WAVE 1 and WAVE 2 (see fig below). These two waves can meet/superpose in two different ways, as shown –
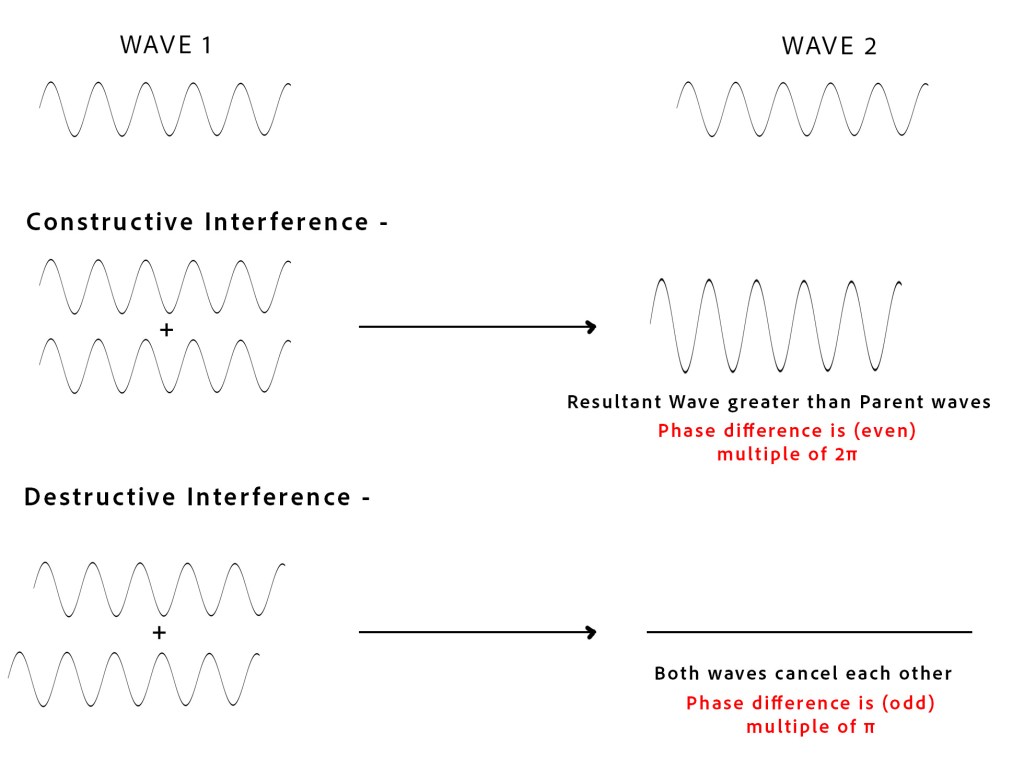
WAY 1 – In constructive interference, the crests and troughs of both waves meet, and the resultant wave is greater than both parent waves.
WAY 2 – In destructive interference, the crest of one wave aligns with the trough of the other wave. Thus, the crests of all the waves automatically align with the troughs of the other wave and vice versa. Hence, both waves cancel each other out.
B] Diffraction –
The bending of a wave around objects is called diffraction. When a wave encounters an obstacle or travels through a slit (small hole), the waves get bent around the corners of that obstacle or spread out after passing through the slit.
Have you seen a CD in the sunlight? We see different colours of light. This is due to diffraction! The light comes in contact with the surface of the CD and gets diffracted to produce lights of varied colours.

The length of the slit / gap through which the wave passes is inversely proportional to the amount of diffraction.
Smaller the gap, the greater the diffraction .
Check the following link –
https://en.wikipedia.org/wiki/File:Wavelength%3Dslitwidthspectrum.gif
Greater the gap,smaller the diffraction.
Check the following -https://en.wikipedia.org/wiki/Diffraction#/media/File:5wavelength%3Dslitwidthsprectrum.gif
Let us imagine a double-slit scenario. Consider a water tank with a rotating paddle (as shown in the figure below). When this paddle moves, it produces waves in water. If we keep an obstacle/dam in the path of the water waves parallel to the propagation of the waves, two large gaps will be created. Let us assume that the length of these gaps is ‘d’. Note that the dam is equidistant from both the corners of the water tank. Now, the waves encounter an obstacle in a confined space. Thus, they pass through the two gaps formed.
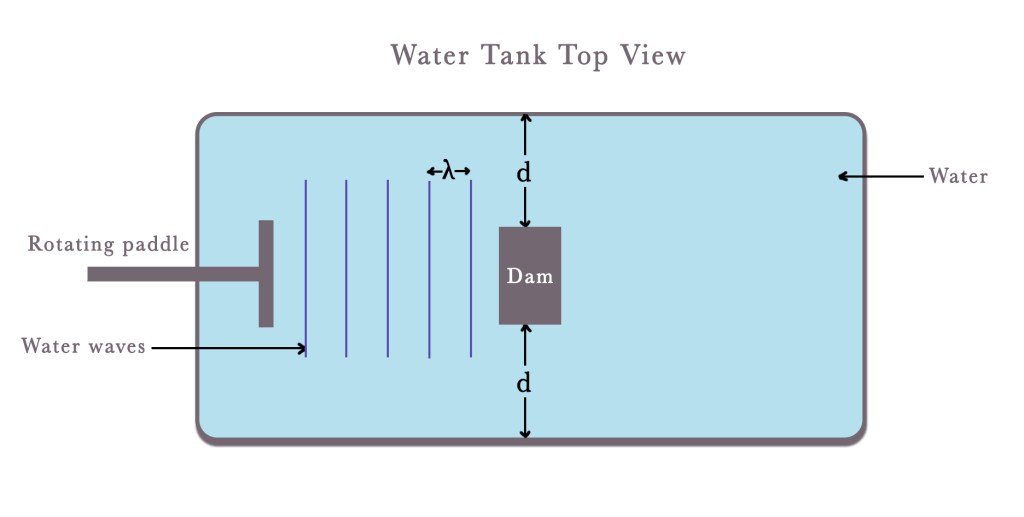
This experiment can have two possibilities –
Possibility 1- d > λ
In the first case, we assume that the length of the gaps, ‘d’, is greater than the wavelength of the created waves, ‘λ‘.
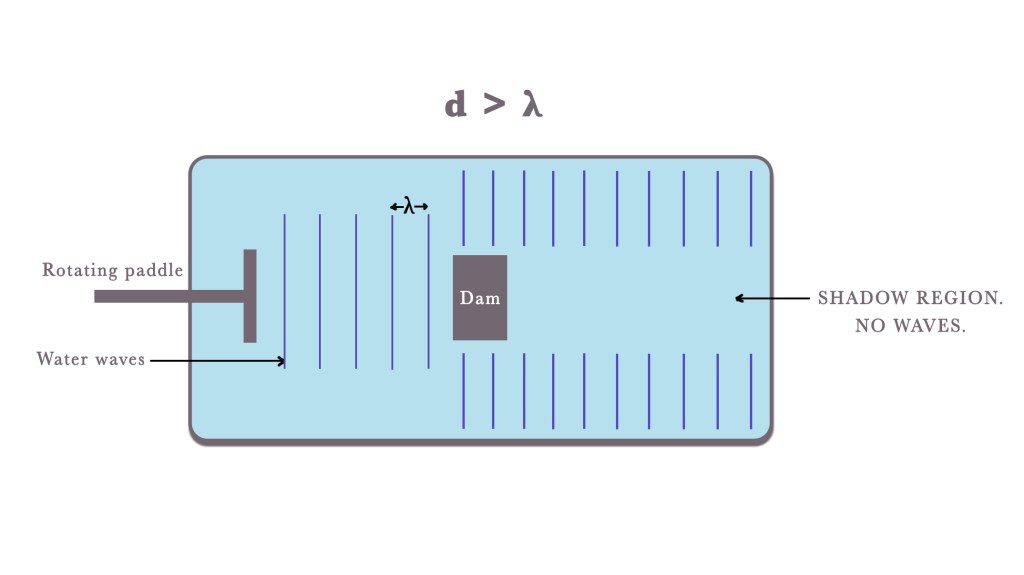
In this case, the obstacle/dam will hinder the transmission of waves and cast a shadow on the other side. The waves will continue to propagate from the sides of the obstacle/dam. However, in the region beyond the obstacle or dam—on the opposite side of the paddle—no waves will be visible, as the barrier blocks their transmission. This is the shadow region. This is comparable to Ray Optics, where the shadow of an opaque object is formed when light waves fall on it.
[NOTE – RAY OPTICS describes the propagation of light in terms of rays.e.g. rays of sunlight.The general assumptions are –
- Rays travel in straight lines in a given medium.
- Rays are a beam of particles.
- Rays split in two when they encounter a hindrance.
- Rays can be reflected from a surface.
- Rays do not exhibit phenomenon of diffraction or Interference as their wave-like nature is not considered.]
Thus, this setup can be modelled as a “water beam system,” where water behaves like a beam of particles—travelling in straight lines and forming a shadow when it encounters an obstacle, similar to how light behaves in geometric optics.
Possibility 2 – d < λ
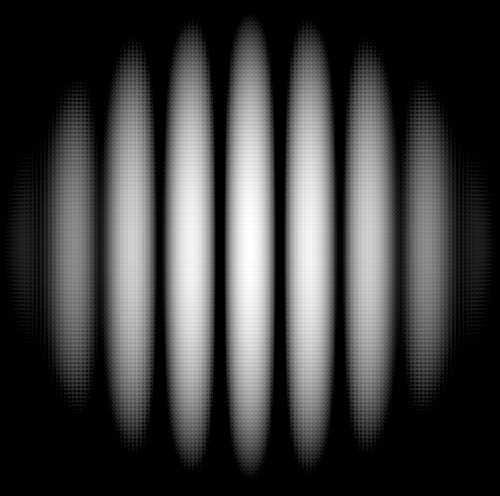
If the spacing between the slits ‘d’ is much less than the wavelength (λ) of the water waves, a different phenomenon is observed. The waves diffract and interfere with each other, giving a characteristic pattern of interference fringes, i.e alternate light and dark regions. THIS IS A PHENOMENON CALLED DIFFRACTION. Here, we do NOT get a shadow; instead, the whole screen has alternate light and dark regions.
This kind of behaviour cannot be explained by ray optics. ONLY WAVE-LIKE BEHAVIOUR OF WATER CAN CAUSE SUCH A PHENOMENON. Thus, we can infer that the water particles behave as waves. They interfere with each other, resulting in constructive and destructive interference patterns, which appear as alternating bright and dark regions on the screen. Diffraction occurs with all waves, including sound waves, water waves and electromagnetic waves such as visible light, radio waves, X-rays, etc. The results obtained are as follows-
After understanding the basics, let us now explore the actual experiment, which served as experimental proof of de Broglie’s thesis.
The Davisson-Germer Experiment
Two American physicists, Clinton Davisson and Lester Germer, were conducting experiments at Bell Labs in New Jersey to study the structure of Nickel by directing a beam of electrons at its surface.
They were shooting accelerated electrons at Ni metal and studying how these electrons were bouncing off the Nickel’s surface. The experiment was conducted in a vacuum chamber to ensure that the electron beams directly hit the target. On one occasion, during their study, some air entered the vacuum chamber. As a result, an oxide film was formed on the surface of Nickel. To correct this, they heated the nickel target to a very high temperature in an oven. This accidentally led to the formation of large single-crystal regions on the surface. Unaware of this change, Davisson and Germer repeated their experiment—and observed unexpected diffraction patterns with distinct peaks!
They later suspected that a change had occurred in the nickel target and began investigating further. This led them to realise that the formation of single-crystal regions was responsible for the diffraction patterns, prompting them to pursue research in that direction.
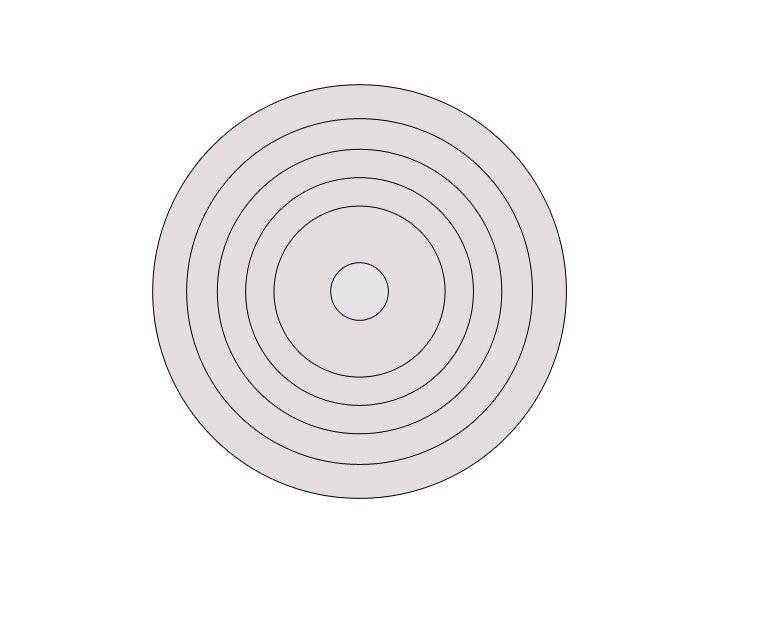
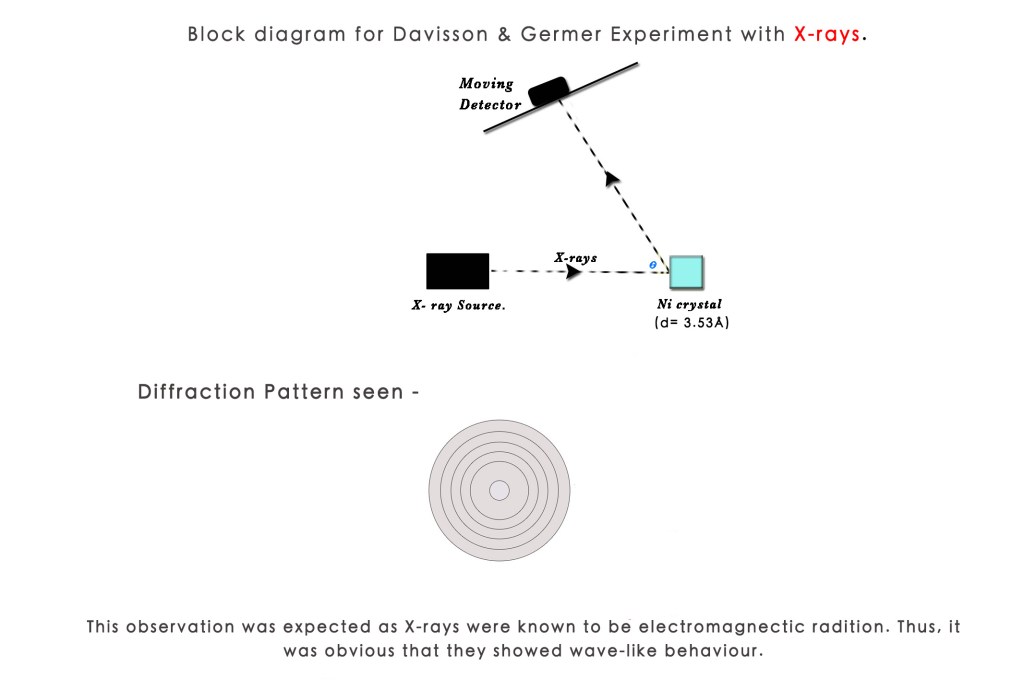
They first irradiated the Ni crystal with X-rays. X-rays are a form of electromagnetic radiation with wavelengths typically around 10 Ångströms (Å). The distance ‘d’ between two atoms in a NiCl crystal is 3.53Å. So, as seen earlier, since the interatomic spacing ‘d’ is less than the wavelength λ, it was expected that a diffraction pattern would form. This pattern typically consists of concentric circles, similar to the ones observed in X-ray diffraction experiments, indicating wave-like behaviour and interference effects.
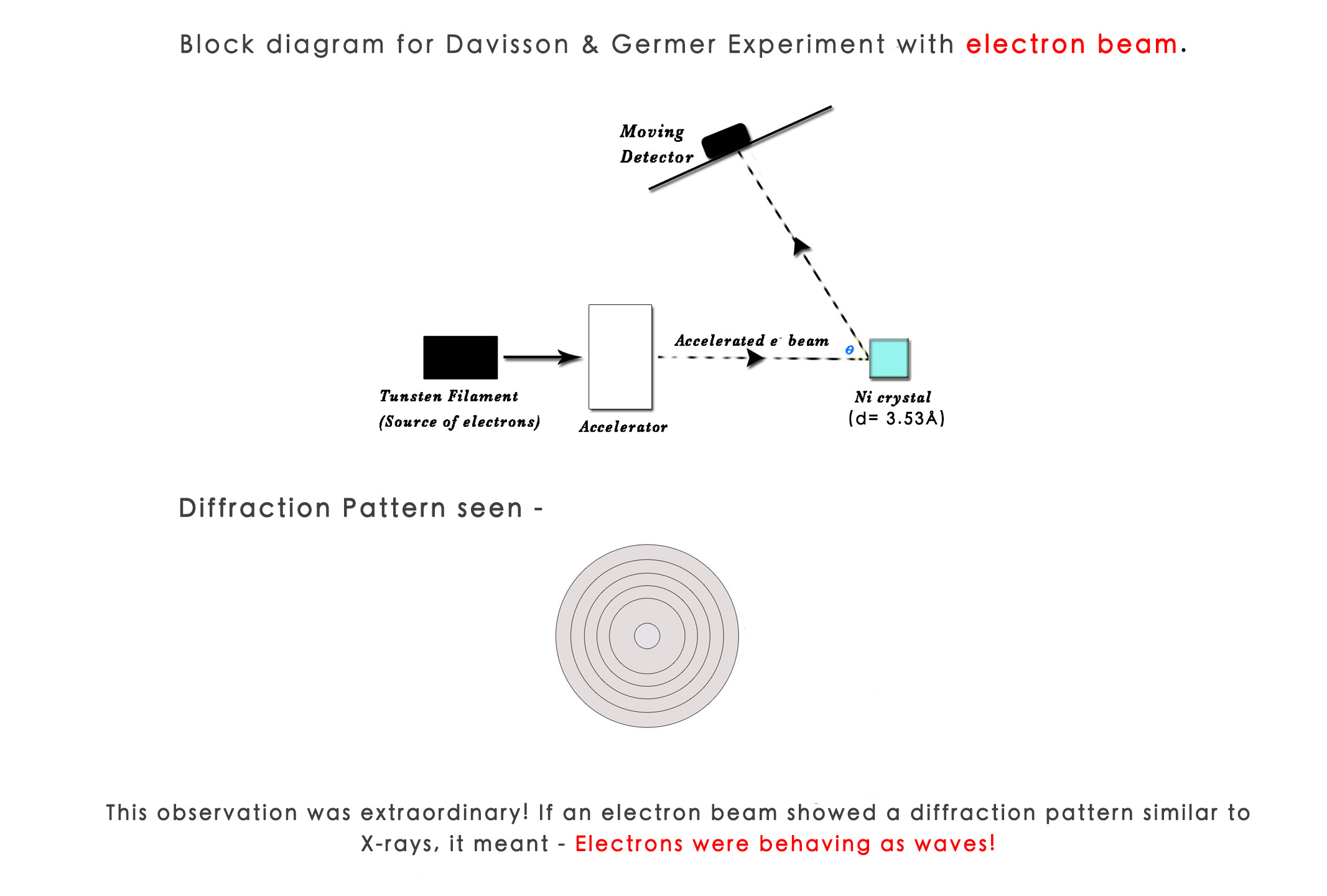
They conducted the same experiment with an electron beam.The source of electrons was a tungsten filament.The ballistic (free) electrons produced, were accelerated and made to hit the Nickel Chloride (NiCl) crystal.Theoretically the wavelength of an electron is 12Å .The result obtained was a diffraction pattern similar to the X-ray diffraction pattern! There was no way of explaining this result, except accepting that the electron beam was behaving as a wave! Thus, this experiment proved experimentally the De Broglie’s hypothesis that matter also behaves as waves.
Note that, X-rays were a representative of waves and electrons were a representative of matter.
In 1929, Louis Victor de Broglie was awarded the Nobel Prize.The Nobel committee stated that –
“When quite young, you threw yourself into the controversy raging over the most profound problem in physics. You had the boldness to assert, without the support of any evidence whatsoever, that matter had not only a corpuscular nature but also a wave nature. Experiments came later and established the correctness of your view.”
Thus, wave-particle duality became an accepted truth in the scientific community, marking a major breakthrough in nuclear and quantum physics. However, if matter behaves as waves, why don’t we observe this in our everyday lives? What else did research in atomic physics uncover? Were there more groundbreaking discoveries? In our next post, we’ll dive into the work of another giant in modern physics and explore his revolutionary thesis.
Till then,
Be a perpetual student of life and keep learning!
Good day !
References and Further Reading –
1)https://en.wikipedia.org/wiki/Interference_(wave_propagation)
2)https://en.wikipedia.org/wiki/Geometrical_optics
3)Lecture 6,MIT Open courseware 3.091.
4)http://abyss.uoregon.edu/~js/21st_century_science/lectures/lec13.html
Image Source –
1)https://en.wikipedia.org/wiki/File:Waventerference.gif
2)http://www.uh.edu/engines/two_slits-b.jpg