In the previous chapters, we explored the concept of electron spin. We began by introducing the idea of spin and then clarified that electrons do not literally spin like tiny balls. We delved deeper into angular momentum, examined how electron spins are measured, and discovered that spin is a quantized property.
In this post, we will build on that foundation and take a closer look at electronic transitions—how electrons move between energy levels and how spin plays a role in these processes.
We know that when an electron absorbs photons of suitable energy, it jumps from the ground state to a higher electronic state. This process is called the excitation of the electron. Depending upon the energy of the photon absorbed, the electron can jump from the ground state to any higher electronic state.
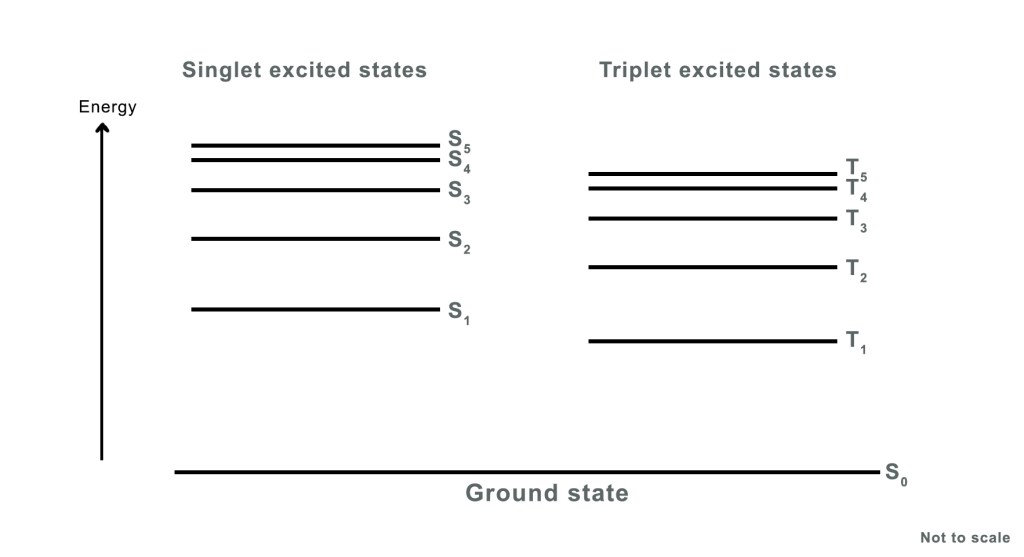
Thus, an electron can transition from the ground state (S₀) to higher singlet excited states such as S₁, S₂, S₃, S₄, and so on. This gives rise to a series of singlet excited states, collectively denoted as Sₙ, where n = 1, 2, 3, 4, etc. This occurs when the electron does NOT undergo a spin change during excitation.
S₁ ⇒ first singlet excited state
S2 ⇒ second singlet excited state
S3 ⇒ third singlet excited state
If the electron changes its spin, we have a series of triplet excited states, Tn, where n=1,2,3,4, etc. T₁ is usually the lowest-energy triplet state and most commonly involved in photophysical processes like phosphorescence.T₂, T₃, etc., are higher triplet states. They are less commonly accessed. However, they can be important in energy transfer and advanced photochemical mechanisms.
Quantum mechanical calculations show that a singlet excited state has higher energy than the corresponding triplet excited state. Thus,
Esinglet > Etriplet
∴ES1 > ET1
ES2 > ET2
ES3 > ET3
The energy level diagram for these two excited states looks as follows-
Why is the triplet more stable than the singlet excited state?
In the ground state, most molecules are singlets (all electrons paired). When an electron is promoted to a higher orbital, i.e when it gets excited, two major factors come into play –
1) Hund’s Rule of Maximum Multiplicity –
Electrons prefer to occupy different orbitals with parallel spins before pairing up. This is because the parallel spins reduce electron–electron repulsion. If two electrons have parallel spins, they cannot occupy the same region of space in the same orbital. Thus, the electrons are more spatially separated. This reduces the Coulombic repulsion between them.
2) Exchange Energy (Stabilisation) –
As the Coulombic repulsions are less in the triplet state, the energy of the triplet state is naturally lower than that of the singlet states. The difference between the two energies is the exchange integral K.
Esinglet− Etriplet = 2K
Thus, the exchange energy favours the triplet state because it promotes a spatial separation of electrons, minimising repulsion.
| Aspect | Explanation |
|---|---|
| Lifetime | Triplet states are long-lived (microseconds to seconds), unlike singlet states (nanoseconds). |
| Phosphorescence | Emission from T₁ to S₀ is called phosphorescence, which is slower and longer-lasting than fluorescence. |
| Reactivity | Molecules in triplet states are often highly reactive, participating in important reactions like photosensitization. |
| Energy Transfer | Triplet energy can be transferred to other molecules, leading to secondary reactions. |
The following post will provide a detailed analysis of the diagram above and introduce further principles of photochemistry. Till the next post,
Be a perpetual student of life and keep learning…
Good day!