In this post, we continue our discussion from the earlier post and delve deeper into the subatomic domain. The plum pudding model, hypothesised by Sir J.J. Thompson failed to correctly explain the structure of an atom. However, his efforts didn’t entirely go in vain. Thanks to his student Ernest Rutherford – a genius who unleashed the power of an atom, by discovering its true structure.

Ernest Rutherford was born on 30th August 1887, in New Zealand. He grew up in a small town in New Zealand on a farm! In 1894, he earned a scholarship, which enabled him to attend Trinity College in Cambridge. In Cambridge, he got an opportunity to work as a research student under J.J. Thompson! Later he went to McGill University, Montreal, in Canada, as a Professor of Physics.
J.J. Thomson wrote supporting Rutherford for the MacDonald Chair at McGill University:“I have never had a student with more enthusiasm or ability for original research than Mr Rutherford and I am sure that if elected he would establish a distinguished school of Physics at Montreal.”
While at McGill University in Canada, he discovered that the α – particle is formed due to the disintegration of a parent nucleus into daughter elements. Thus he found nuclear transmutation. He won a Nobel Prize in Chemistry in 1908 for this discovery.
Rutherford left Mc Gill university in 1907 as he thought that Europe was a better place to conduct his research.He stated –
“I shall be glad to be nearer the scientific centre as I always feel America as well as Canada is on the periphery of the circle.”
He came to Victoria University of Manchester, England. As a Nobel laureate, Rutherford determined that the α – particles are helium nuclei (He2+) – helium atoms devoid of electrons. Thus he concluded that they are positively charged. The β – particles are negatively charged electrons (e–). In 1919, he accepted an invitation to succeed his mentor sir J.J.Thompson, as the Cavendish Professor of Physics at Cambridge.
THE Gold Foil / Rutherford-Geiger-Marsden EXPERIMENT



In 1907, an important research series began under Rutherford’s guidance. Rutherford and Hans Geiger were studying deflections of α- particles in electric and magnetic fields.
The experimental setup had a ZnS screen, which would produce a scintilla of light when the α- particles hit it. Spots were produced on the screen where the α-particles hit it. They observed that there was a change in the deflection pattern of the α-particles when a mica sheet was introduced in its path. In this case, the spots on the photographic plate became blurred. They saw this phenomenon each time the mica plate was inserted. They concluded that the atoms of the mica sheet were scattering the α-particles.
Rutherford and his group of scientists started studying this phenomenon to find an explanation for these observations. They inserted plates of various metals (Al, Pb, etc.) in the α-particle’s path. One day they inserted a gold foil in the path of the α-particles. This became their best-known experiment – The Gold Foil /Rutherford-Geiger-Marsden experiment!
Rutherford and his two associates – Hans Geiger and Ernest Marsden (Marsden was just 20 years old. A college dropout recruited by Rutherford to work in his lab) were using the α- particles to check the validity of the plum pudding model and to find out the reasons behind their observations. They aimed a beam of α- particles at a piece of gold foil of 600 nm thickness.
They choose gold metal because –
1) It is a noble metal. Gold does not corrode. It is not very reactive and is not easily affected by acids. It does not oxide in the air.
2) Gold is highly malleable – it can be drawn into fragile sheets (600 nm/0.6µm thick)
The experimental setup
The source of α- particles was Radium (Z= 88), Polonium(Z=84), and Thorium(Z=90). These elements are highly radioactive and disintegrate to form α- particles. The elements were stored in a lead (Pb) container. Lead has a high density and a higher atomic number. Thus, it acted as a shield and protected the environment from radioactive radiation. The particles were zooming out of this source at 7.68 MeV per particle.
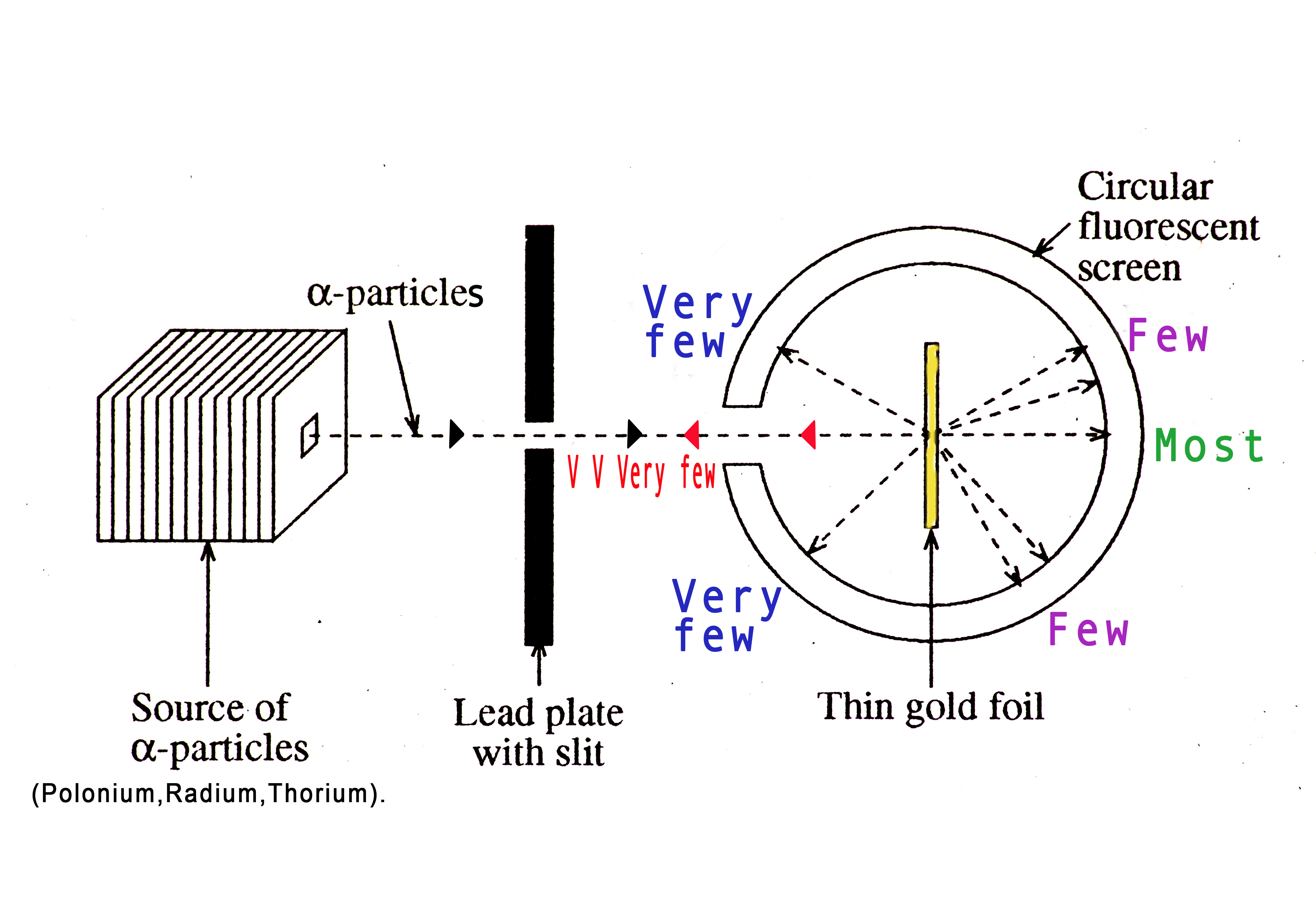
They placed a circular fluorescent screen coated with zinc sulphide (ZnS) to detect the α- particles that pass through the gold foil. This screen was developed by Geiger. The experiment was conducted in total darkness. Each time an α- particle hit the screen, a scintilla (spark) of light was produced. A microscope placed above the screen would magnify the spark making it easy to spot it. Geiger and Marsden kept observing the screen. They would sit alone in total darkness and count the sparks manually!
If the structure of an atom was as the model proposed by J.J. Thompson, all α- particles should have passed through the gold foil and hit the screen directly behind the foil.
Months passed but Geiger and Marsden did not see anything unusual. One day, Geiger bumped into his mentor Rutherford in the corridor outside the lab and reported that they still hadn’t seen anything unusual. Rutherford asked Geiger to check if they saw any scintilla of light on the same side as that of the experimenter. He suggested that they place the screen on the same side of the source! Rutherford was a man of intuition! His students followed his instructions and did exactly what he asked them to do. So changed the position of the screen and placed it on the same side of the source.
Observations
- Most α- particles behaved exactly as expected. They passed directly through the foil, without any deviation and hit the screen behind the foil.
- A few α- particles were deflected at small angles (by 1 or 2 degrees).
- Very few were deflected by an angle of 90 degrees.
- For days after the suggestion by Rutherford, Geiger and Marsden saw absolutely nothing. One afternoon they saw a scintilla of light on the screen on the same side of the source. Geiger reported to Rutherford in great excitement that – ‘We have been able to get some of the α-particles coming backwards!’ Rutherford quoted –
“It was quite the most incredible event that has ever happened to me in my life. It was almost incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you”. (Rutherford, 1938, p. 68)
This was a great discovery! They found out that very few α-particles (1 in 20000) were deflected at an angle of 360 degrees from the parent beam! The particle fired right back at the experimenter! This phenomenon of ‘backscattering’ led to the discovery of the nucleus!
The Plum pudding atomic model could not explain these observations. Thus, Rutherford proposed his own nuclear model. He gave credit to Geiger too.
“Geiger is a good man and worked like a slave. I could never have found time for the drudgery before we got things going in good style. Finally, all went well, but the scattering is the devil. Our tube worked like a charm and we could easily get a throw of 50 mm for each particle. Geiger is a demon at the work of counting scintillations and could count at intervals for a whole night without disturbing his equanimity. I damned vigorously and retired after two minutes”. (Quoted in Eve, p. 180.)
Rutherford’s Atomic Model
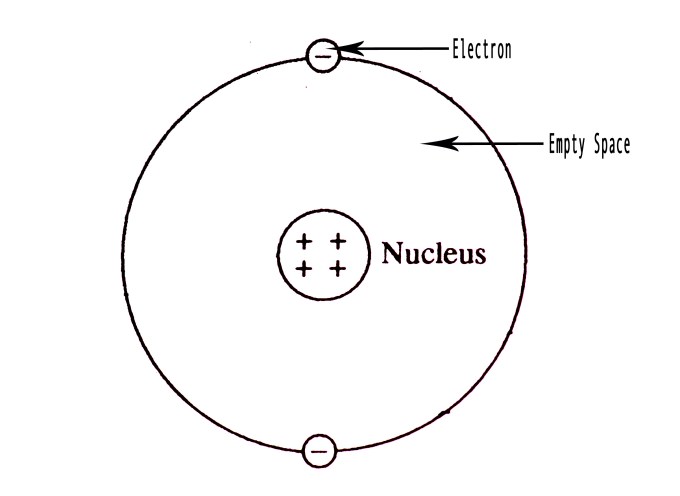
Based on his experiments, Rutherford concluded that –
♦ An atom must be extremely hollow and must have empty space within it as most α- particles pass through it without any deviation.
♦ The number of negatively charged electrons dispersed outside the nucleus is the same as the number of positively charged in the nucleus. It explains the overall electrical neutrality of an atom.
♦ The α- particles would be deflected by small angles, if they got close to the positive centre, due to electrostatic repulsion.
♦ Very few particles fling back at the source. These α- particles must be encountering a huge positive charge from which they get repelled completely. However, as the frequency of these particles (deflected at 360 deg) is low, this positive charge must occupy a very little volume as compared to the volume of the atom. He thus inferred that an atom consists of a heavy positively charged body at its centre called the nucleus. The nucleus contained protons. This atomic model was also referred to as the Nuclear model. The nucleus occupies a small fraction of volume of the atom. All the weight of the atom is concentrated in the nucleus. Electrons have negligible mass as compared to that of protons.
♦ The electrons revolve around the nucleus in circular orbits, with huge velocity. This is comparable to the planetary motion around the sun. Thus, this model is also called the planetary model.
♦ Marsden calculated the diameter of the nucleus. He found out that-
the radius of the nucleus/Radius of the atom ≈ 1/10000
the diameter of a nucleus is around 10-15 m.
the diameter of a nucleus is around 10-10 m.
Thus, there is lots of empty space in the atom. This explains why most α- particles went zooming through the gold foil without getting deflected.
To understand this ratio, assume that one takes out all the empty space from all atoms in a human body. The human will be reduced to just a speck of dust without the void! However, the weight would remain the same!
If the empty space from all atoms in all the human bodies in this world is taken out, the remaining mass would be the size of an apple! Another way to imagine this is the nucleus is just a spec of a dust particle in an atom which is as huge as a cathedral!
Limitations of Rutherford’s planetary atomic model
Rutherford’s atomic theory received a lot of condemnation from the scientific world. The reason for this strong negative reaction was that the theory could not explain the following points –
♦ The electrons moving around the nucleus are negatively charged. So, due to Coulombic/electrostatic forces of attraction, the electrons should get attracted to the nucleus and there should be a NUCLEAR COLLAPSE! However, this never happens.
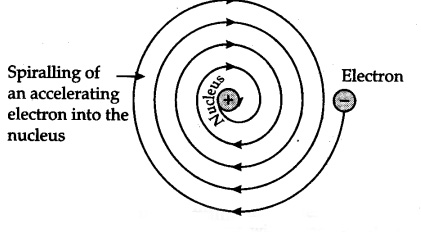
♦ According to classical mechanics and electromagnetic theory, any moving ,accelerated charged particle emits electromagnetic radiation(EMR) and thus lose energy. Thus, the electrons should spiral into the nucleus – BAM !! or just stop due to ENERGY DEFICIET !! Although in reality an atom is very stable and electrons keep moving around the nucleus.
♦ Another drawback of Rutherford’s model was that it did not say anything about the arrangement of electrons in an atom. This made his theory incomplete. He assumed that the electrons could occupy any position around the nucleus. This arrangement should give us a continuous spectrum. However, an atomic spectrum is a line spectrum with discrete lines. Thus, this theory was unable to explain the atomic spectra too.
So, what happened next? Was the Rutherford model correct? Did it need amendments? Who would decode this mystery? We shall find the answers to these questions in the upcoming post. Till then,
Be a perpetual student of life and keep learning…
Good day !
References and Further Reading –
1.http://www.nobelprize.org/nobel_prizes/chemistry/laureates/1908/rutherford-bio.html
2.http://myweb.usf.edu/~mhight/goldfoil.html
3.https://www.aip.org/history/exhibits/rutherford/sections/alpha-particles-atom.html
4.https://en.wikipedia.org/wiki/Ernest_Rutherford
5.MIT 3.091SC Introduction to Solid State Chemistry, Fall 2010 by Professor Donald Sadoway,Lecture 3.
6.History of atom (BBC) documentary.
Image source –
1.https://en.wikipedia.org/wiki/Geiger-Marsden_experiment#/media/File:Ernest_Rutherford_LOC.jpg
2.By Unknown – http://wal.nbed.nb.ca/sciencesettechnologies/pierrebrideau/geiger.jpg, Public Domain, https://commons.wikimedia.org/w/index.php?curid=34187140
3.By S P Andrew Ltd. – http://mp.natlib.govt.nz/detail/?id=27055&l=en, Public Domain, https://commons.wikimedia.org/w/index.php?curid=34174345
4.http://www.chemistryland.com/CHM130S/05-EarlyAtom/EarlyAtom.html#
5.http://ask.learncbse.in/t/give-the-drawbacks-of-rutherfords-atomic-model/4432
Lovelyy post
LikeLike