THE NOBEL PRIZE.
The Nobel Prize for Chemistry this year is awarded to three great chemists. They are Jean-Pierre Sauvage (University of Strasbourg, France), Sir J. Fraser Stoddart (Northwestern University, USA), and Bernard L. Feringa (the University of Groningen, the Netherlands) for “the design and synthesis of molecular machines“. They developed the world’s smallest machines – molecules with controllable movements! These can perform tasks when energy is added to them. These new developments are bound to lead to many technological advancements. Let us thank all the inventors who help make our lives better by harnessing the magic hidden within atoms!
This post is an extension to our discussion on the atoms previously. In the 19th century, this infinitesimal part of the matter was revealing its secrets like never before! Scientists were going deeper to find the world within this tiny space. The discoveries were mind-boggling and the complexity of the structure of an atom was beyond imagination! Efforts to correlate the atomic structure with the classification of various elements also gained momentum.
With the advent of the concepts of four quantum numbers and sub shells, new questions were raised. How do electrons fill in these orbits? Is it a random event or is there a pattern to it? Along came many theories, four of which became the basis of our understanding of the periodic table today.
Pauli’s exclusion principle
Wolfgang Pauli proposed the first theory. He was a theoretical physicist who enunciated a theory known as Pauli’s exclusion principle. It states –
No two electrons can have the same set of quantum numbers in an atom.
No two electrons in an atom have the same 4 quantum Numbers. Electrons in the same orbital have the same values of three quantum numbers n,ℓ, and m. However, the value of the spin quantum number for every electron differs. This is because if one electron in the orbital is moving in a clockwise direction, the other has to move in an anti-clockwise direction. Thus, they will have spin quantum numbers s= +1/2 and s= -1/2, respectively.
e.g. In a helium atom, there are two electrons in the 1s orbital. For both the electrons , n=1,ℓ = 0 and m=0. But one of them has s= +1/2 (spin up – ↑ ) and the other, s= -1/2(spin down – ↓) .
Aufbau principle
‘Afbau’ is a German noun that means ‘construction‘ or ‘building-up‘. This principle is not named after any scientist. It was formulated by Niels Bohr and Wolfgang Pauli. It explains how the electrons fill up in various shells and sub-shells. It states that,
In an atom, the electrons fill the orbitals of lower energy first, and only then the subsequent higher energy orbitals are filled.
Thus, the electrons will always prefer to enter a lower energy orbital.
e.g.– 1s is lower energy than 2s orbital. Thus, the electron in the hydrogen atom will occupy the 1s orbital.
The Madelung (n+ℓ) energy ordering rule
In 1931, Erwin Madelung, a German physicist, proposed an empirical rule for filling the orbitals with electrons. According to this rule,
The orbital with minimum (n+ℓ) value gets filled first by electrons.
e.g. Let us consider 4s and 3d orbitals.
For 4s, (n+ℓ) = 4+0 = 4 (as ℓ = 0 for a s- orbital – as it has spherical shape)
For 3d, (n+ℓ) = 3+2 = 5 (as ℓ = 2 for d-orbital).
Thus, the 4s orbital will be filled first by electrons. Therefore, the order of filling the electrons in orbitals is 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d. This can be represented in two ways as follows-


Hund’s rule of maximum multiplicity
This rule throws light on the possibility of electron pairing in an orbital.
Consider two electrons of equal energies wanting to occupy degenerate orbitals ( two orbitals with the same energies). Will the two electrons pair up in a single orbital or occupy different orbitals?
One can think of an analogy here. Consider two benches in a garden. If two people want to sit on those benches, will they sit on the same or different bench? Hund’s rule of multiplicity explains this phenomenon. It states that,
The electrons do not pair up in degenerate orbitals until each orbital is first singly filled with parallel spin.
NOTE – AN ORBITAL CAN ACCOMMODATE A MAXIMUM OF TWO ELECTRONS ONLY.
Thus, orbitals of equivalent energy strive for an unpaired electron. Orbitals prefer to have as many unpaired electrons as possible before starting the pairing.
e.g. – Nitrogen (Atomic number 7) has three electrons in a 2p orbital.
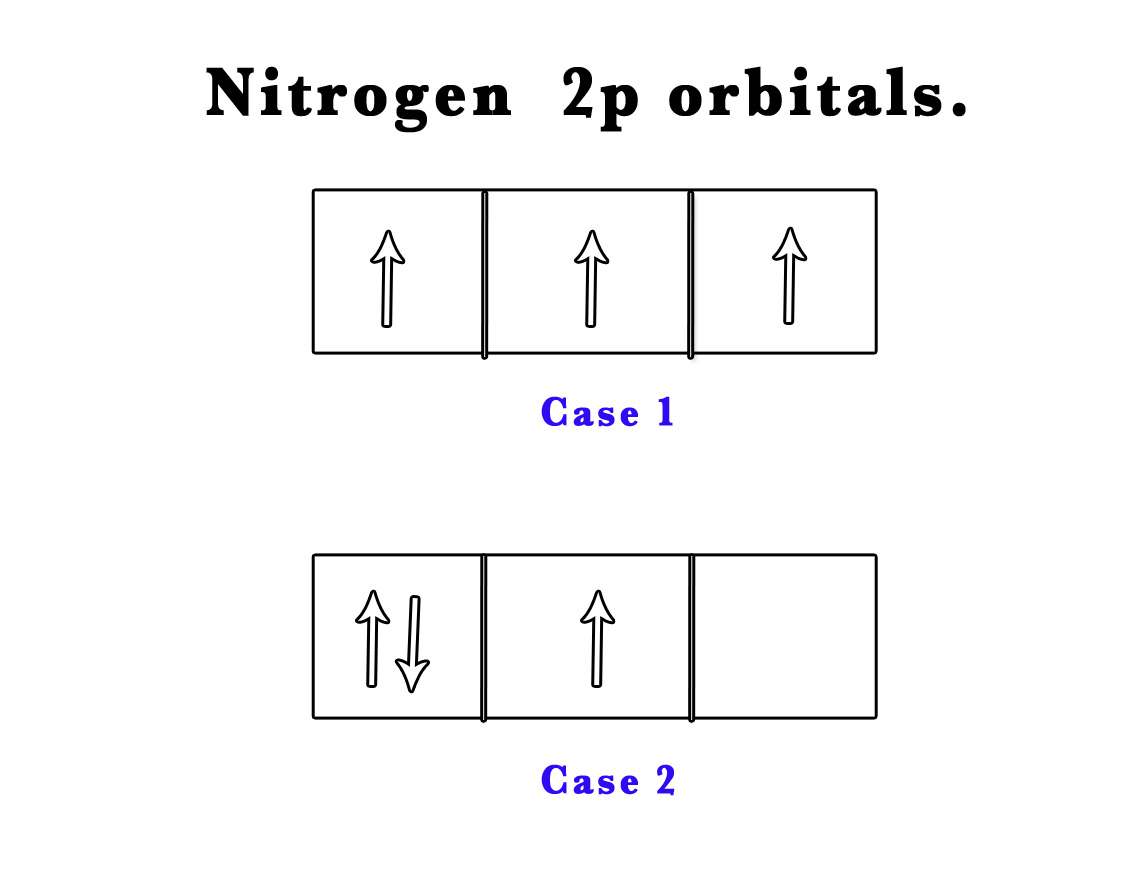
Case 1 – the three electrons can occupy three different orbitals ( 2px, 2py and 2pz) and thus not pair up at all.
OR
Case 2 – two electrons can pair up in a single orbital (one spin up and another spin down) and the third can later occupy the next orbital.
According to Hund’s rule of maximum multiplicity Case 1 is likely to be observed.
These theories helped scientists correctly classify elements in the periodic table. The properties of elements could now be studied based on the electron distribution within the atom. This distribution of electrons was called the ‘electronic configuration‘ of that element.
e.g. The electronic configuration of Helium is 1s2 which indicates that there are 2 electrons in the s-orbital of the first shell.
The electronic configuration is the basis of everything we know today, as it dictates the properties of elements! The electronic configuration of any element can be derived by using the fundamental rules stated above.
Question – Write the electronic configuration of carbon (Atomic number= 6).
Answer -1s2 , 2s2,2p2.
(Practice writing the electronic configurations of various elements from the periodic table).
The story of the atom doesn’t end here .. it continues.. more on that in my next posts. Till then,
Be a perpetual student of life and keep learning…
Good day!
References and further reading –
1)https://www.nobelprize.org/nobel_prizes/chemistry/laureates/2016/press.html
2)https://en.wikipedia.org/wiki/Aufbau_principle
One comment