In the 19th century, the research on the structure of the atom was yielding incomprehensible results. Scientists worldwide were astounded by the revelations. However, this also stoked up the desire to find out more about the atom and thus the nature of the universe.
It is remarkable though that amidst this research, a very fundamental particle – the neutron was not discovered till 1932! In 1920, Ernest Rutherford pointed out the disparity between the atomic number and the atomic mass of atoms.
Why does Helium (Z=2) have mass four times that of hydrogen (Z=1)? It was known that electrons don’t have significant mass. So all the mass of an atom comprises the mass of its protons. If Helium has 2 protons and hydrogen has 1, then ideally, the mass of helium should be double that of hydrogen. So, why is it that helium is four times heavier than hydrogen?
Mass of helium = 4×(Mass of hydrogen)…WHY?
Thus, Rutherford conceptualized that there was a possibility of the existence of some other particle, which is electrically neutral and is a component of the nucleus. However, there was no solid evidence to prove that.

It was in 1932, that James Chadwick, an English Physicist, discovered this sub-atomic particle!
In his undergraduate days(1911), James Chadwick was Rutherford’s student at Victoria University of Manchester. In 1913, he was awarded a research scholarship and he went to Berlin, Germany, to study under Hans Geiger. The first world war broke out in 1914, and James Chadwick became a prisoner of war in Germany. When the war ended, after 4 years, he went back to his mentor, Ernest Rutherford in Cambridge University. In 1921, Rutherford made him assistant lab director at the Cavendish lab at Cambridge University. In 1932, he was awarded the noble prize for the discovery of neutrons!
James Chadwick, who was working with Rutherford, was assigned the task of finding out the neutral particle, which Rutherford had predicted earlier.
In 1931, Walther Bothe and Herbert Becker discovered that when α-particles hit a target of a lighter metal like Beryllium, Lithium, or Boron, they emitted strong penetrating radiation. This radiation was first thought to be gamma rays but later it became evident that it was something else. This radiation was far more penetrating than gamma radiation.
Irene Curie and her husband discovered that when a beam of this radiation hit a substance rich in protons, for example, paraffin(an alkane), protons were knocked off from it, which could be detected by a Geiger counter.
James Chadwick started his experiments in this direction to find out the nature of this radiation. The experiment he conducted was as follows-

As shown in the above figure, he used Polonium as a source of α- particles. Polonium (a radioactive element) emits α- particles, which then hit the beryllium foil. This led to the emission of some kind of radiation. This emitted radiation was not deflected by an electric field. Thus, it had to be electrically neutral. This radiation was very penetrating. Thus, it knocked off the protons(H+) from the paraffin slab. As soon as these protons (H+) enter the gas-filled chamber, they ionised the gas within. The recorder, a Geiger counter, recorded the amount of gas ionized, which was proportional to the number of protons entering the chamber.
Thus, James Chadwick proposed that it was the neutral particle present in the atom’s nucleus, which is causing this effect on the paraffin. he concluded that this particle was the one, whose presence, Rutherford had predicted earlier. Thus, he discovered the neutron! The nuclear reaction that produced neutrons was as follows –
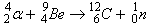
Later he went on to find the mass of this neutron. He observed that the mass of a neutron was nearly equal to that of the proton(slightly larger than the proton mass). Thus, three fundamental particles of the atom were discovered – THE PROTON, THE ELECTRON, and THE NEUTRON.
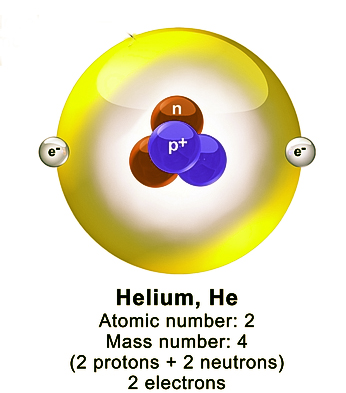
It could now be fully understood, why the mass of the Helium atom was 4 times the mass of the hydrogen atom. The helium atom consists of 2 protons and 2 neutrons, which accounted for its mass. The electrons revolve around the nucleus as shown below-
So, was this the final structure of the atom? Was this an inerrant model? Let us find the answers to this question in the next post. Till then,
Be a perpetual student of life and keep learning…
Good Day!
References and further reading –
1) https://en.wikipedia.org/wiki/Discovery_of_the_neutron
2)https://sites.google.com/site/chadwickexperiment/home
3)http://www-outreach.phy.cam.ac.uk/camphy/neutron/neutron5_1.htm
4)http://www.physicslab.org/Document.aspx?doctype=3&filename=AtomicNuclear_ChadwickNeutron.xml
Image source –
1)https://en.wikipedia.org/wiki/File:James_Chadwick.jpg
2)http://www.physicslab.org/Document.aspx?doctype=3&filename=AtomicNuclear_ChadwickNeutron.xml
3)By BruceBlaus – Own work, CC BY 3.0, https://commons.wikimedia.org/w/index.php?curid=33041232