Bonding in molecules forms the basis of elucidating their structure, which in turn dictates the properties of substances. In this post, we shall learn about the second kind of primary bond – The Covalent/Molecular bond.
P2. THE COVALENT BOND.
This bond is formed due to the sharing of electron pairs between atoms. The atoms involved in covalent bond formation have the same/similar electronegativity.
Watch this video to understand how the sharing takes place-
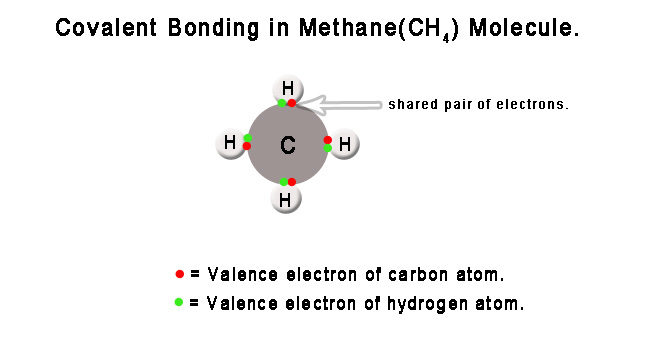
e.g. – A methane(CH4) molecule is formed when electrons are shared between one carbon and four hydrogen atoms. The sharing ensures that the carbon atom’s octet(8 electrons in the valence shell) and hydrogen atom’s duplets(two electrons in the valence shell) are complete. In general, the octet and duplet(for hydrogen and helium atoms) render stability to atoms. In this case, it makes carbon and hydrogen atoms more stable, and thus, a new molecule is formed.
There are mainly three theories explaining the formation of covalent bonds –
1) Valence bond theory (VBT),
2)Hybridization theory, and
3)Molecular orbital theory(MOT).
The first two theories help explain the shape and overlapping of orbitals and the latter theory describes the molecular orbitals in detail. These three theories together, give us a clear picture of bonding in covalent bonds in a classical sense. Quantum mechanics offers a very different view of bonds. Let us study these theories one by one.
VALENCE BOND THEORY (VBT).
This theory was first postulated by Walter Heitler(who studied under Arnold Sommerfield and worked with Neils Bohr in Copenhagen and Erwin Schrodinger in Zurich) and Fritz Wolfgang London(‘London dispersion forces’ fame, 5 times nominated for Nobel prize in Chemistry) in 1927.


This theory is based on certain assumptions which are termed ‘the postulates of valence bond theory‘.
The postulates of the VBT
The postulates of VBT are –
- Condition for bond formation – A covalent bond is formed when adjacent atomic orbitals of two atoms containing unpaired electrons overlap. These unpaired electrons must have opposite spins.
- The binding force of the bond – Chemical bonds form when two atoms approach each other and the attractive forces are greater than the repulsive forces.
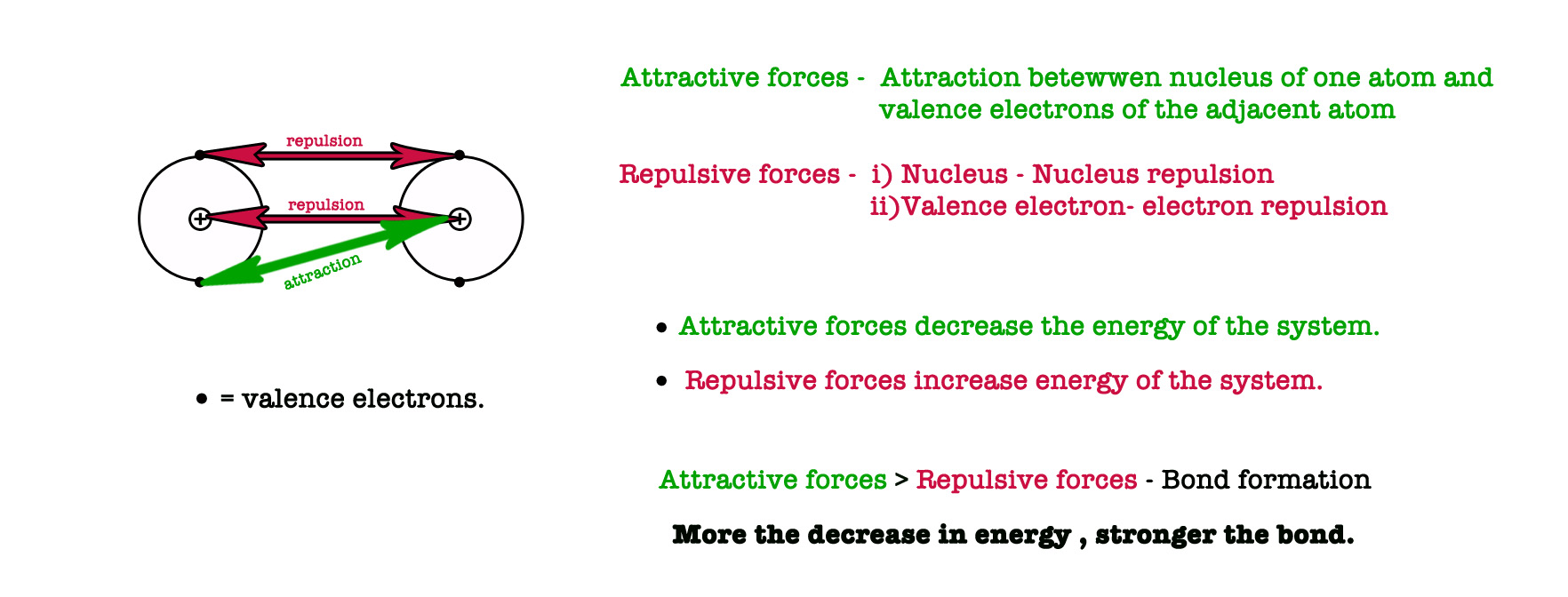
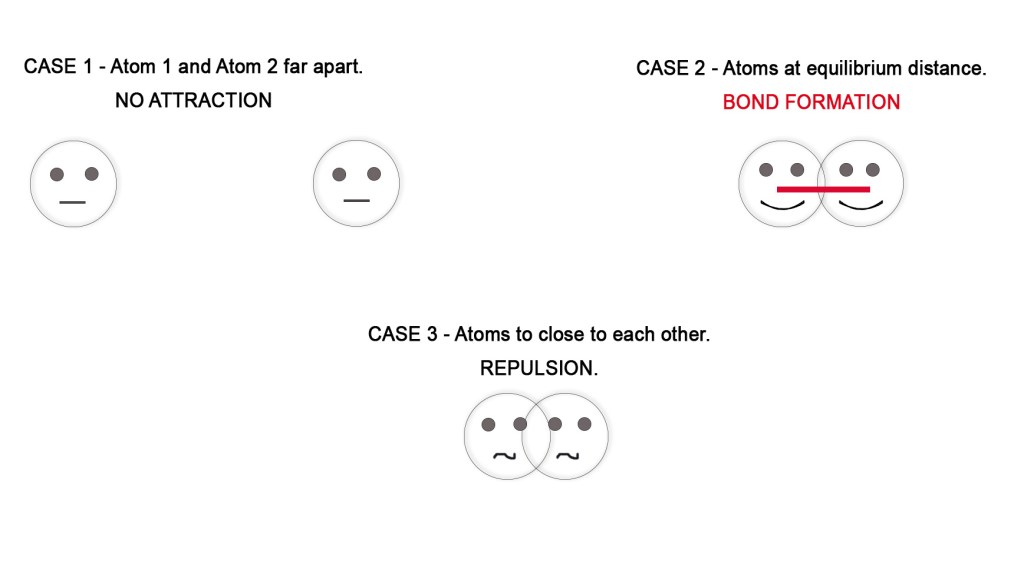
After the overlap, the electron density increases in the region of the overlap i.e in the region between the nuclei of two atoms. Due to this, the repulsion (like charges repel each other) between two nuclei is reduced and the attraction between the nuclei and the valence electrons increases. - Stability of the system – Whenever repulsive forces decrease and attractive forces increase, there is a decrease in the energy of the system. This is because the attractive forces bind the molecule together and render stability to the system (more stability = decrease energy). The overlapping takes place only at equilibrium internuclear distance, where the repulsion is minimum. If the atoms come more closer than the equilibrium distance, the repulsive forces increase making the bond less stable.
4. Energy of the overlapping orbitals – The energies of the overlapping orbitals should be nearly the same.
5. Strength of the bond – Greater the overlap, the stronger the bond.
6. Number of covalent bonds formed –
# of bonds formed = # of unpaired electrons .
e.g.- Hydrogen has 1 unpaired electron so it forms one bond, oxygen has two unpaired electrons so it can form two bonds. Nitrogen has 3 unpaired electrons so it forms 3 bonds.
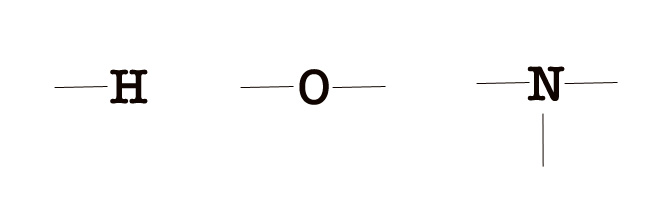
7. Directional nature of the bond – We have already learned that s- orbitals are non-directional (as they are spherical). However, p-, d-, and f- orbitals have a particular shape and orientation in space. Thus, the geometry of the molecules depends on the orientation of the overlapping orbitals. The orbitals are oriented in a way where they are as far away as possible from each other, ensuring minimum repulsion and maximum symmetry.
The valence bond theory helps reveal how overlapping takes place in molecules. Let us discuss in the next post how exactly this happens, with the help of examples. Till then,
Be a perpetual student of life and keep learning …
Good day!
References and further reading –
1.https://en.wikipedia.org/wiki/Valence_bond_theory
Image source –
- By GFHund – Own work, CC BY 3.0, https://commons.wikimedia.org/w/index.php?curid=11197534
- https://www.goethe.de/ins/ie/en/kul/sup/dsi/20817070.html