sp3d3 hybridization
The process of mixing one s- orbital, three p- orbitals, and three d-orbitals in an atom to form seven sp3 d3 hybrid orbitals of equivalent energy is called sp3 d3 hybridization.
EXAMPLE 1 – IODINE HEPTAFLUORIDE (IF7)
Iodine is a p-block element and it has 7 valence electrons. Both iodine and fluorine are halogens and thus iodine heptafluoride is an interhalogen compound – a compound formed between two halogens. In this compound, iodine is the central atom that undergoes hybridization.
Iodine (53) 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p5 → Ground state
One s and two 5p electrons get excited to the vacant d-orbital, which gives rise to 7 unpaired electrons. The 5s,5p and 5d orbitals hybridise to form seven sp3 d3 hybrid orbitals.The molecule is then formed with 7 fluorine atoms.

The geometry of this molecule is pentagonal bipyramidal. Five F atoms are in one plane directed at corners of a pentagon (equatorial position), with a 72º angle between them. One F atom is above and one F atom below the plane of the pentagon(Axial positions). The angle between axial and equatorial F atoms is 90º.
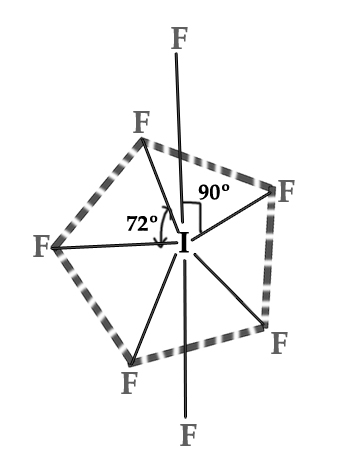
The 3D structure can be represented as follows –
sp3d4 hybridization
This type of hybridization is very rare as it demands the central atom to be bonded to eight different atoms/ligands. Very few elements show an oxidation state of more than +7 (e.g.- Mn+7). We shall not study this in detail as its occurrence is very rare.
The key points to remember about the hybridization theory are –
• # of atomic orbitals mixed = # of atomic orbitals obtained.
• The electrons in unhybridized p orbitals form π bonds.
• Hybrid orbitals form more stable bonds than pure atomic orbitals.
• The hybrid orbitals are always equivalent in energy and shape.
• The type of hybridization depends on how many atomic orbitals are mixed (this depends on the reaction conditions).
|
No.of substituents on the central atom |
No. of hybrid orbitals |
Hybrid orbitals |
Type of hybridisation |
Ideal Geometry |
Bond Angle/s |
|
7 |
7 |
one s + three p + three d |
sp3d3 |
Pentagonal bipyramidal | 72º, 90º |
|
6 |
6 |
one s + three p + two d |
sp3d2 |
Octahedral |
90º |
|
5 |
5 |
one s + three p + one d |
sp3d |
Triagonal bipyramidal (TBP) | 120º, 90º |
|
4 |
4 |
one s + three p |
sp3 |
Tetrahedral |
109.5º |
|
3 |
3 |
one s + two p |
sp2 |
Triagonal Planar |
120º |
|
2 |
2 |
one s + one p |
sp |
Ⅼinear |
180º |
With this post, we complete the study of all types of hybridizations. In the next post, we will study a new rule, which is important in our study on hybridization theory. Till then,
Be a perpetual student of life and keep learning…
Good day!!
Image source –
