Molecules with hybrid orbitals can be of two types –
A] Symmetric molecules where all bonds are the same.
e.g.- CH4 molecule, where all bonds are C-H bonds. So, in this type of molecule, all substituents on the central atom are the same. This type of molecule show ideal bond angles and bond lengths as predicted by the VSEPR model.
B] Asymmetric molecules, where all the substituents on the central atom are not the same.
e.g.- NH3 molecule, where nitrogen is bonded to three hydrogen atoms and has a lone pair too.
OR
Water molecule with two lone pairs of electrons has sp3 hybridized oxygen atom.
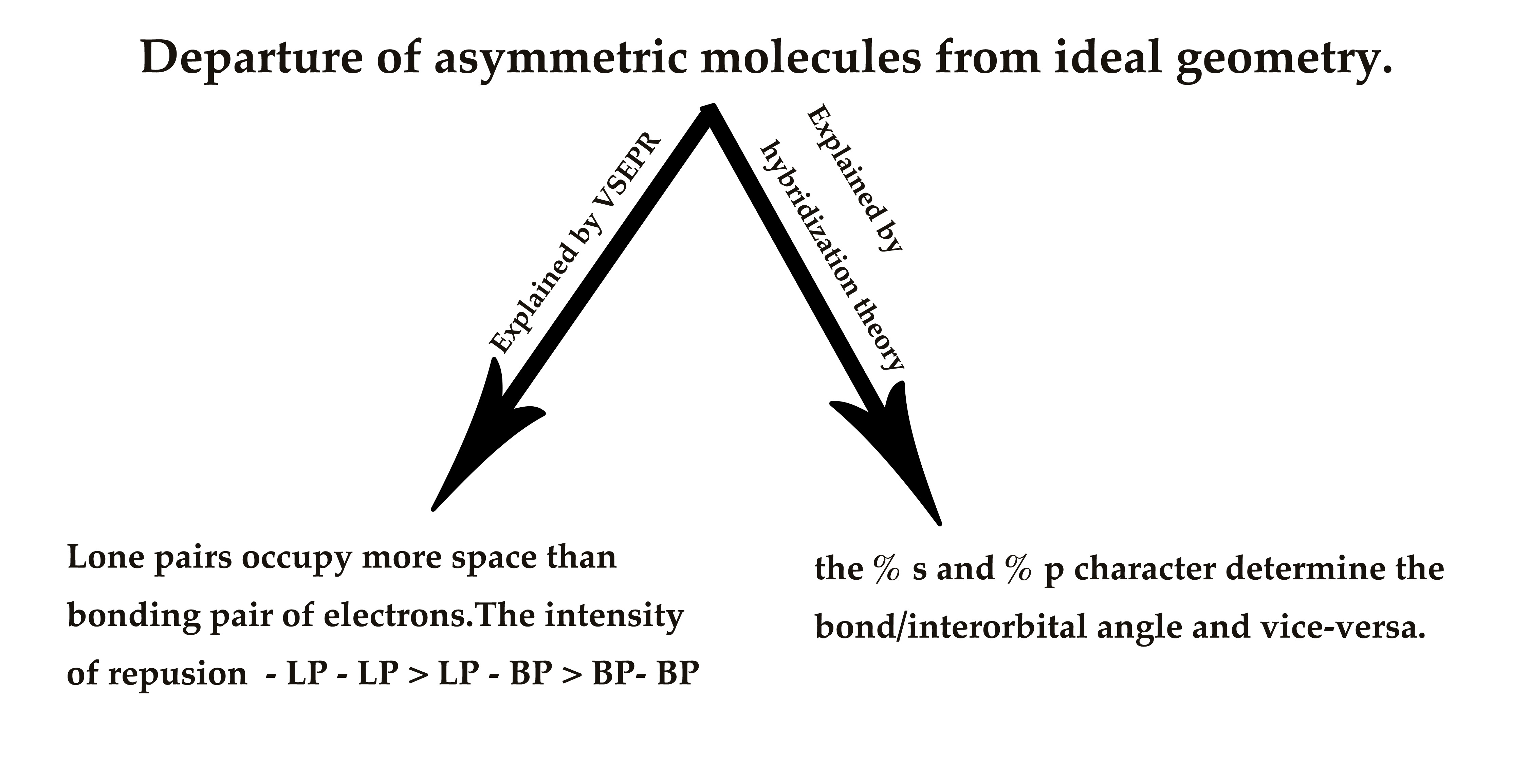
Asymmetric molecules show the departure from ideal behavior, as predicted by VSEPR.
We have already studied, how the VSEPR theory, explains this departure from ideal bond angles and bond lengths. It does so, by stating that the lone electron pair occupies more space than the bonding pair of electrons. However, hybridization theory explains the same non-ideal behavior in a different way. Hybridization theory relates the %s and % p character to bond angles.
Let us study in detail how the %s character and bond angles are related.
From the last post we already know,
i= fp / fs , where,
i ⇒hybridization index.
fp ⇒ fraction of p character.
fs ⇒ fraction of s character.
For hybrid orbitals containing s and p orbitals, the sum of f values for all the hybrids on a given atom must be equal to 1.
∴fs +fp = 1
e.g.- In sp3 hybridization, fs +fp= 0.25+0.75= 1
For all hybridization, s =1, as there is only one valence s orbital, which takes part in the mixing process.
We also know that the total no of hybrid orbitals formed = i + 1
∴fs= 1 / (i + 1) and fp= i/(i+1) → (eqn 1)
Applying the above formula to sp3 hybridization , i= 3 .So,
fs= 1 / (3 + 1) = 1/4 = 0.25
fp= 3 / (3 + 1) = 3/4 = 0.75
According to hybridization theory, the % s and % p character determine the bond/interorbital angle (i.e the angle between two hybrid orbitals ) and vice-versa.
When we plot the f values vs the interorbital angle (θ), we get the following graph –
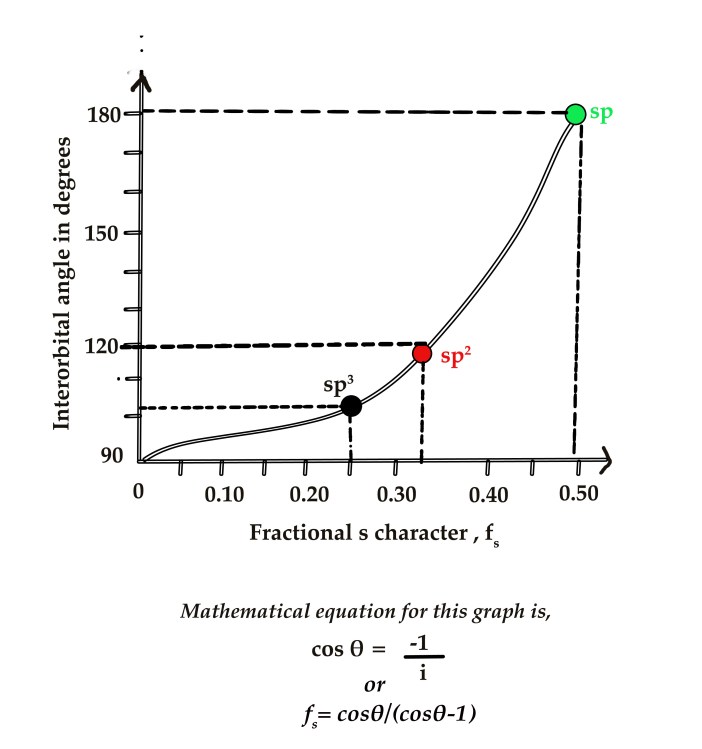
As seen in the above graph ,
for sp3 hybridization , i=3 , fs = 0.25 (i.e 25% s character) , and θ sp3 = 109.5 º
for sp2 hybridization , i=2, fs = 0.33 (i.e 33% s character) and θ sp2 = 120 º &
for sp hybridization , i=1, fs = 0.50 (i.e 50% s character) and θ sp = 180 º.
The above graph yields the relation,
cos θ = -1 / i or fs=cosθ/(cosθ-1) – This is a mathematical representation of the graph, which is beyond our course of study. So, we shall just take the formula as it is.
Let us check, if the above equation, cos θ = -1 / i gives us the correct values of θ.
i) For sp3 hybridization , i=3 , cos θ = -1 / i .
∴cos θ = -1 /3
θ = cos-1 ( -1/3) = 109.5º
ii)For sp2 hybridization , i=2
∴cos θ = -1 /2
θ = cos-1 ( -1/2) = 120º
iii)For sp hybridization , i=1
∴cos θ = -1 /1= -1
θ = cos-1 (-1) = 180º
It’s just amazing to see how mathematics, theoretically proves, the observations made experimentally !
Relating fs to θ
The bond angle (θ) is related to fs value by the following two equations –
fs=cosθ/(cosθ-1) or cosθ = fs/ fs-1
Also, the summation of all fs values for a given atom should equal to one, as only one atomic s- orbital is involved in the hybridization process.
∴∑ fs = 1.
Consider methane molecule(CH4). In this molecule, the central carbon atom is sp3 hybridized. It has 4 hybrid orbitals. Each sp3 hybrid orbital will have f(s)=0.25. However, carbon has 4 such orbitals.Thus, for the atom,
∑f(s) =0.25+0.25+0.25+0.25= 1.
Remember the entire s-orbital is used to make hybrid orbitals.

We continue this discussion in the next post as well. Till then,
Be a perpetual student of life and keep learning ..
Good Day!
References and Further Reading –
1.http://www.acadblock.com/inorganic-chemistry/inorganic-comprehension-ft64/
2. Organic Chemistry by Roger Macomber, Volume 1.
Thank you very much for an awesome post; it really cleared a lot of confusion for me.
But, I’d like to ask how the summation of fs can =1 (as written in the last point of formulae to remember). Wouldn’t it be more accurate to say that the sum(fs)=1/(i+1)?
LikeLike
Hi Raima.. I am so glad you found my post helpful… f(s) = 1/(i+1). However, the summation of f(s) has to be equal to 1. To help you understand, let me give you an example – Consider methane molecule(CH4). In this molecule, the central carbon atom is sp3 hybridized. It has 4 hybrid orbitals. Each sp3 hybrid orbital will have f(s)=0.25. However, carbon has 4 such orbitals.Thus, for the atom,summation f(s) =4(0.25)= 1.
Remember the entire s-orbital is used to make hybrid orbitals.
LikeLike