Before starting to solve problems on the mole concept, let us jot down a few important formulae –

Let us solve some problems based on these formulae.
1)Calculate mass of one molecule of carbon dioxide.(At. wt of C = 12 , O=16).

A:
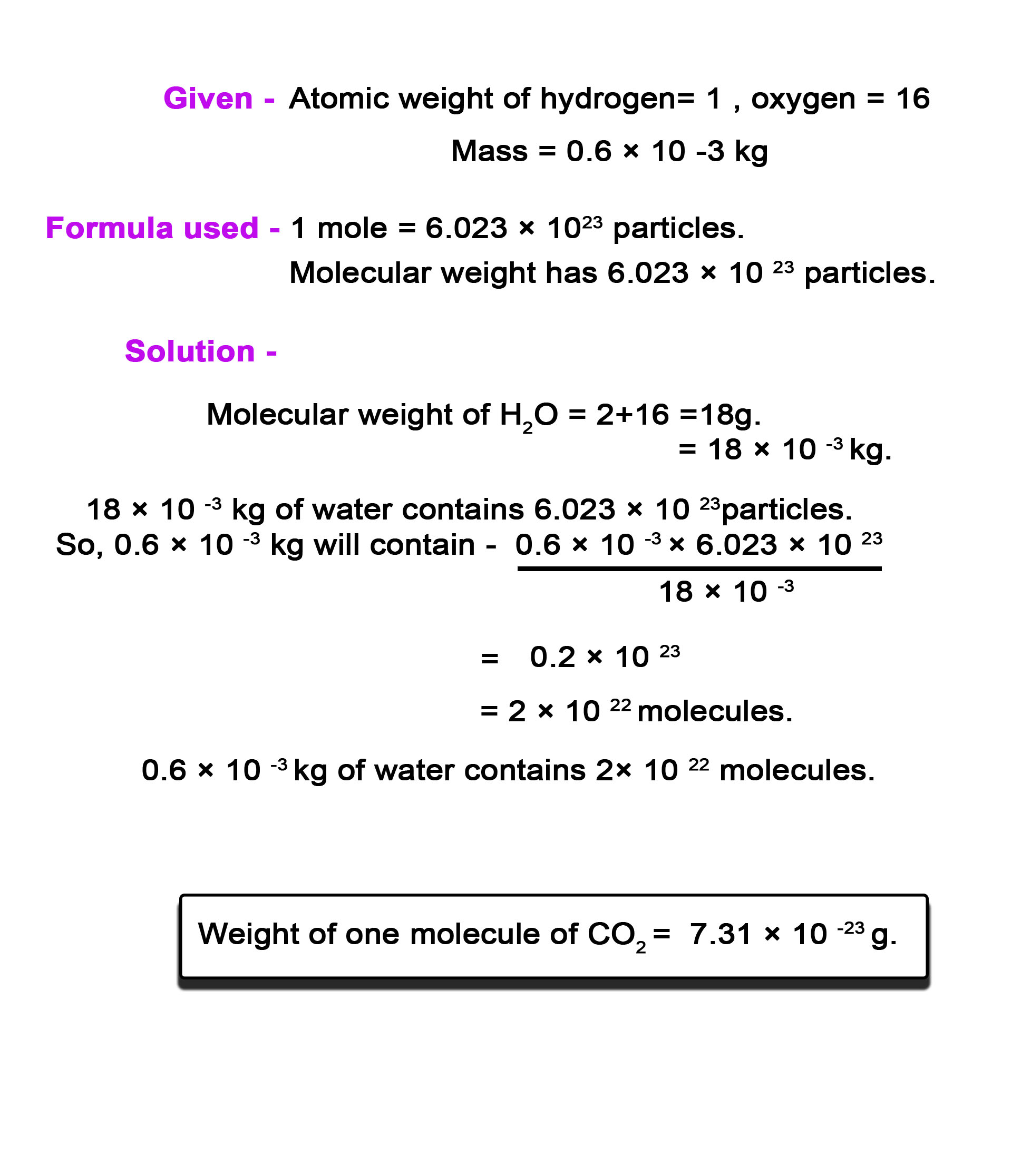
2) Calculate the number of molecules present in one drop of water having mass 0.6 × 10 -3 kg. (At wt of H = 1 , O= 16) ,
3) Calculate the weight of 0.4 mole of NaCl.

In the next post we will learn some new concepts useful in analytical calculations. Till then,
Be a perpetual student of life and keep learning….
Good day !