BASES
In general, a base is a compound that yields OH– ion/s on dissociation.

Example 5 – Let us now consider a base, sodium hydroxide (NaOH). This compound will dissociate in water as follows –

In this case, we have OH– ions instead of H+ ions . 1 mol of NaOH will produce 1 mol of OH– ions. Therefore, the acidity of this base = nf =1.
(From the periodic table→ At.mass of Na =23, At.mass of O=16,At.mass of H=1)
Molecular weight of NaOH = 23 +16+1= 40g/mol.
Equivalent weight = 40/1=40 g/eq.
∴ the Eq.Wt of NaOH = M.Wt of NaOH.
Example 6– Calcium hydroxide Ca(OH)2.
The dissociation of calcium hydroxide yields two moles of hydroxide ion(OH–) .
 Thus, in this case, the n-factor is 2.
Thus, in this case, the n-factor is 2.

The acidity of a base is defined as the no of ionizable hydrogen ions (OH-) present in one molecule of a base. However, the n=factor is NOT always equal to its acidity. The n-factor depends on the reaction in which the base is participating.
For the above reaction,
M.Wt of Ca(OH)2= 40+ 2(16+1)=74 g/mol.
Eq.Wt of Ca(OH)2 = M.wt/2 = 74/2 = 37eq/mol.
∴ the Eq.Wt of Ca(OH)2 = M.Wt/2.
Example 7- Aluminium hydroxide Al(OH)3.

As seen above, the dissociation of aluminum hydroxide yields three moles of hydroxide ion(OH–).
Thus, in this case, the n-factor is 3.However, the n-factor can be 1 or 2 too.
M.Wt of Al(OH)3= 27+ 3(16+1)=78 g/mol.
Eq.Wt of Al(OH)3 = M.wt/3 = 78/3 = 26eq/mol.
∴ the Eq.Wt of Al(OH)3 = M.Wt /3.
ATOMS AND IONS
n-factor for atoms is their valency. Thus, this term is also called as the valence factor. Every element has one or multiple valencies depending upon their position in the periodic table. This valency is considered as the n-factor of these elements. How to find valency for an element?
Generally, s- and p- block elements have a fixed valency. The transition metals have multiple valencies. Let us have a look at the periodic table –
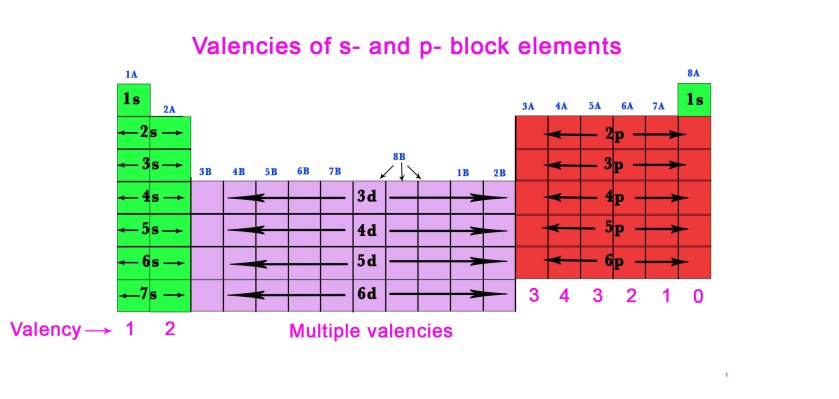
As seen in the above periodic table,
- the transition metals(d-block elements) have multiple valencies i.e they exhibit various oxidation states.
e.g.- In Cr2O3 chromium has a valency of +3. Whereas, in K2Cr2O7 the valency of chromium is +6. We all know about iron, which forms ferrous(Fe+2) and ferric (Fe+3) ions.
Thus, for transition metals, the oxidation state in the compound is the n-factor.
- The elements in group 1A have only 1 electron in their valence shell (ns1)and so they exhibit +1 valency.
e.g.- Na+ , K+ , Rb+ etc - Similarly, elements in group 2A have two electrons in their outermost shell. So, they form cations with 2 charges by donating those two electrons. They have +2 valency. e.g.- Mg2+,Ca2+ etc
- Group 3A and 4A have +3 and +4 valency respectively as shown in the figure above. e.g.- Al3+ etc.
- Group 5A elements(Pnicogens) have 5 valence electrons. These elements have either +3 or +5 valency. However, nitrogen exhibits nine different oxidation states!
e.g.– Nitrogen has a -3 oxidation state in NH3, whereas it is seen to have a +5 oxidation state in HNO3. Phosphorous has a +5 oxidation state in PCl5. - Group 6A and 7A have valency 2 and 1 respectively as it is easy for these elements to gain two or one electron/s to complete their octet, rather than loosing 6/7 electrons. e.g.– S2-, O2-, Cl–, Br– etc.
- Group 8A elements have a valency of zero as these are inert gases with a completed octet. e.g.-Ar,Kr etc.
In the next post we will try to see some examples of salts and find their n-factor. Till then,
Be a perpetual student of life and keep learning….
Good Day!