In the previous posts, we have studied in detail, how to find n-factor and equivalent weight for a number compounds. In this post we shall talk more about the actual concentration term- normality.
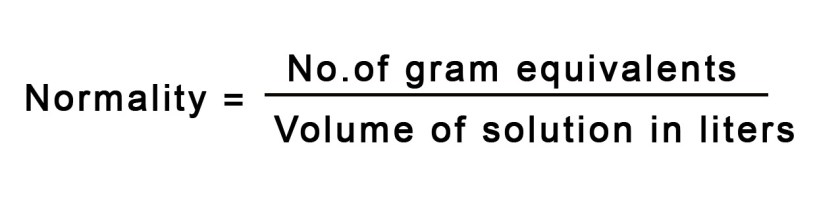
As studied in post #143, normality term gives us the # of gram equivalents per litre of the solution. This means that if we dissolve 1g equivalent weight of a substance to make 1 litre of solution, we would get 1N solution of that substance.
So,
If we dissolve 49g of H2SO4 in 1 litre of water , we will get 1N H2SO4 solution.
If we dissolve 62g of boric acid in 1 litre of water , we will get 1N boric acid solution.
If we dissolve 40g of NaOH in 1 litre of water , we will get 1N NaOH solution.
Relationship between Molarity and Normality
N = nf × M
Normality is not a very frequently used concentration term due to its limitations. The biggest limitation of this term is that it depends on the chemical reaction under consideration. Thus, a solution of H2SO4 has a fixed molarity but normality depends on how it reacts in a reaction!
We use this term in titrimetric calculations.(We will learn those calculations when we study titrations in detail).
5.Weight,Volume and Weight to volume ratios
There are many units under this section which express the concentration in weight to volume ratio-
| No. | Concentration unit | Denoted as | Definition | Type |
| 1. | weight percent | %w/w | grams of solute per 100g of solution | Weight to weight ratio |
| 2. | Volume percent | %v/v | mL of solute per 100mL of solution | Volume to volume ratio |
| 3. | weight to volume percent | %w/v | grams of solute per 100mL of solution | weight to volume ratio |
| 4. | parts per million | ppm | i)micrograms of solute per gram of solution or ii)milligrams of solute per liter of dolution(aq solutions) | weight to volume ratio |
| 5. | parts per billion | ppb | Nanograms of solute per gram of solution or micrograms of solute per liter of solution. | weight to volume ratio |
6. p-function.
Sometimes during a reaction , a reactant’s concentration may change by many orders of magnitude. In such a case, plotting a graph of the change of concentration as function of time or volume of reagent is a more convenient way to study the reaction. In such cases, we express concentration as a p-function.
p-function of a number X is the negative log of that number.
pX = –log(X)
e.g.-pH = –log[H+] = –log(0.10) = 1.00…the pH of a solution that is 0.10 M H+ is 1.
We can easily plot [H+] (on y-axis) vs volume of Base added to it( on x-axis) and study the reaction graphically! We will see these kind of reactions in the titration chapter, which we shall study soon.
In the next post we shall start solving problems relating to various concentration terms.Till then,
Be a perpetual student of life and keep learning…
Good day !