We talked about different methods of analysis in the previous post and inferred that only with experience and intuition, we can choose the correct way of analyzing the analyte. However, it is of utmost importance that we obtain an accurate representative sample, on which we can conduct the analysis. This post is exclusively dedicated to the concept of sampling in analytical chemistry.
What is a sample?
The word sample has two meanings in analytical chemistry- it can be used as a noun and as a verb!
Sample(n) – Substance to be analyzed which is assumed to be representative of the bulk.
Sample (v) – To collect one or more samples.
Chemical analysis is most often performed only on a small fraction of the bulk, whose composition reflects, as closely possible, the average composition of the bulk/target population. The process by which such a representative sample is acquired is called sampling.
Sample is a finite part of a statistical population whose properties are studied to gain information about the bulk sample.
We know that sometimes harmful substances contaminate water bodies like rivers, lakes,s or a reservoir. Suppose we want to study water pollutants in a river. It is impossible to carry out analysis on the entire river water (bulk) and so it is very important that we obtain some finite part of the river water, from different locations, which can serve as a representative of the river water(bulk sample). This sample water collected should possess all the properties of the bulk i.e it is like a mini replica of the larger population.
Terms involved in Sampling
1. Universe/bulk/Population – This is the total material that needs to be analyzed. For example, if we need to find pollutants in river water, then the river water is our universe or bulk material.
2. Sample – This is a small part drawn from the universe, which represents the bulk, for the purpose of analysis or the process of finding the correct sample.
3. Sampling unit– The minimum sized package from the universe, which represents the sample is called a sampling unit. e.g.– If our consignment contains many boxes then each box is a sampling unit.
4. Increment – A small portion that has been taken directly from the universe without alteration.
5. Gross sample – Increments are taken from different sampling units of the same universe. On mixing form a gross sample. Most of the time, when we mention the term ‘sample’ we mean gross sample.
6. Analysis sample– An accurately weighed quantity of the sample taken for actual analysis is called an analysis sample.
7. Test portion – A portion of a collected sample that is processed and measured.
8. Analyte – The chemical species to be identified or quantitated. This might exist as a pure substance or as one of the constituents of a multicomponent sample.
9. Detector – Device that responds to the presence of the analyte, usually generating an electrical output.
10. Signal – The detector output that is displayed or recorded.
11. Sensitivity– The change in detector signal vs change in analyte concentration.
12. Selectivity – The discrimination of an analyte vs other components in the sample.
How do we sample? Designing a sampling plan.
Sampling procedures are of three types namely – 1)Random Sampling, 2)Systematic sampling, 3)Selective/Judgemental sampling
1)Random sampling – As the name suggests, this type of sampling involves merely selecting samples from the bulk randomly in an unbiased manner. However, randomness doesn’t mean collection in a haphazard manner. It means applying a randomization process to obtain a correct sample from the target population. In this type of sampling, each entity has an equal chance of being chosen. There is no bias in choosing any part of the bulk.
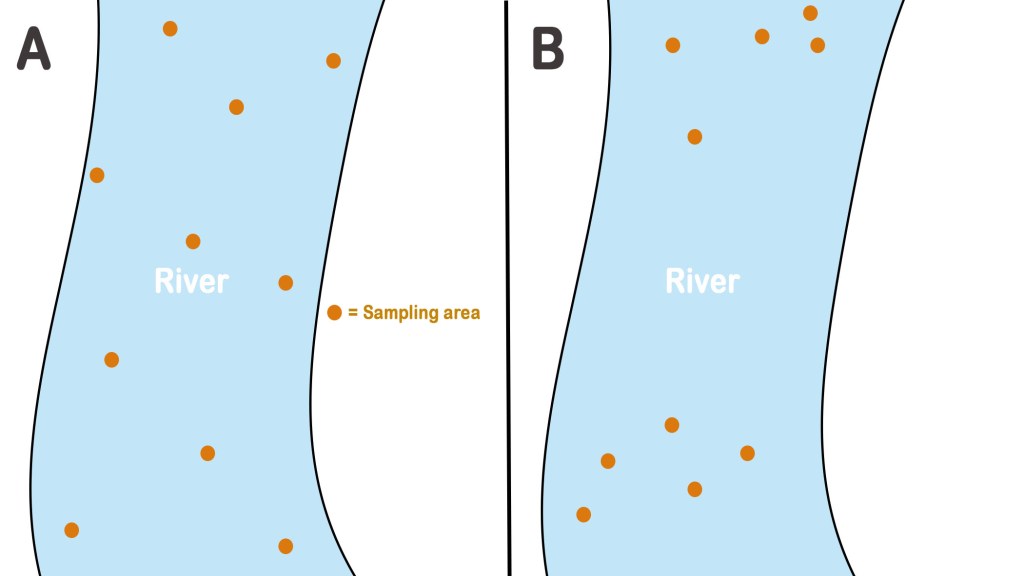
As seen in the picture above, in part A of the picture, the sampling of river water is done randomly but the samples are collected from various points distributed over the entire space. In part B, however, the sampling is done in a haphazard manner and the sample points are not evenly spread over the entire area of the river. Thus, in the case of B, we shall not get a sample, which correctly represents the river water(bulk). The variation in sampling is called sampling error.
Random sampling requires more time and expense than other sampling methods since a greater number of samples are needed to characterize the target population. This kind of sampling is easy when the system is homogeneous. However, in a heterogeneous universe, it is very difficult to randomly sample as the analyte is not homogeneously distributed in the bulk. A simple solution to this problem is homogenizing the bulk. However, this is not a practical solution most of the time. Also, doing so may lead to loss of information about the spatial distribution of the analyte in the bulk. Thus, in such cases, a different approach is needed.
2)Systematic sampling – In this type of sampling, the sample units are drawn in a definite sequence at equal intervals from one another. The sample points from a larger population are selected according to a random starting point but with a periodic interval or sequence.
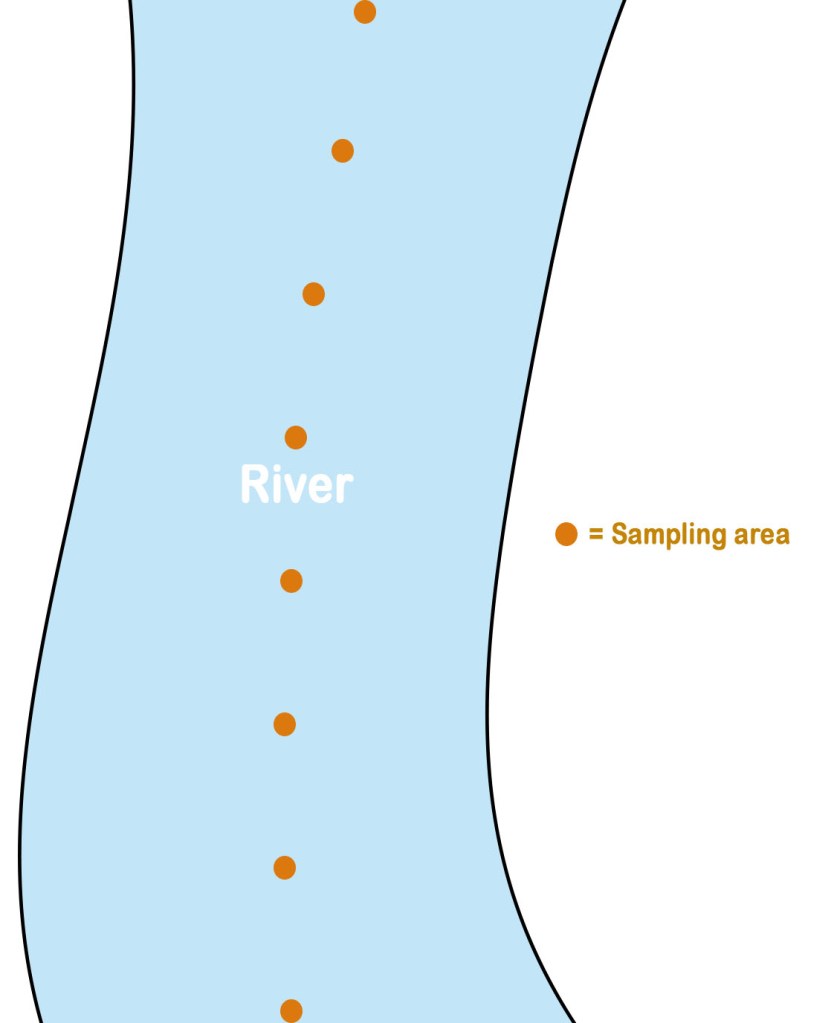
As seen in the above picture, the samples are collected from the center and the sample points are equidistant. Though this type of sampling appears to be more scientific, in reality, it may not give a better sample than one obtained in random sampling.
3)Selective/Judgemental sampling – In this kind of sampling is biased from the analyst’s point of view. Thus, is exactly the opposite of the random sampling technique. In this method of sampling, the analyst uses information about the target population to help select samples.
e.g.– Suppose we want to find out metal content in coins. Then, we can intensionally neglect corroded coins and select only the ones which are not corroded.

In the next post, we shall continue discussing sampling in greater detail. Till then,
Be a perpetual student of life and keep learning….
Good day!
References and further reading –
- Principles of Instrumental Analysis 6th Edition by Douglas A. Skoog, F. James Holler, and Stanley R. Crouch.
2. Basics of Analytical Chemistry and Chemical Equilibria by Brian M. Tissue.