In the previous post, we familiarised ourselves with the concept of electron spin. We learned that spin is an intrinsic property of an electron – something which it has inherently. The electrons don’t actually rotate; however, they still possess a spin. Let us delve deeper into this idea in this post.
What is a spin?
Spin is a type of angular momentum. We know that objects moving in a straight line have linear momentum. The linear momentum of an object can be calculated by multiplying its mass by its velocity.
Linear Momentum(p) = mass (m) × velocity (v)
Angular momentum is the rotational equivalent of linear momentum. Objects that are in an angular motion – rotating/spinning around an axis or revolving – have angular momentum.
The angular momentum quantifies an object’s tendency to continue rotating about a specific point or axis.
More Angular Momentum means
→ Object has greater rotational motion or is rotating faster and/or farther from the axis.
→ Harder to stop or change its direction of rotation.
→ Indicates a stronger tendency to keep spinning.
Less Angular Momentum means
→ The object has a slower rotational motion, or the mass is closer to the axis.
→ Easier to stop or change rotation.
→ Weaker tendency to continue rotating.
Thus, the angular momentum is a physical quantity that reflects an object’s resistance to changes in its rotational motion. The greater the angular momentum, the more strongly the object tends to keep rotating unless acted upon by an external torque.
Mathematically, angular momentum (L) is defined as –
L= I⋅ω
Where,
L = Angular momentum (in kg·m²/s)
I = Moment of inertia
ω = Angular velocity (in radians per second)
Moment of Inertia –
Moment of inertia (I) is the rotational equivalent of mass. It describes how an object’s mass is distributed around its axis of rotation. It’s the tendency to keep rotating unless acted on by an external torque. It describes how difficult it is to change an object’s rotational motion, either to start, stop, or change its rotational speed.
The farther the mass is from the axis, the greater the moment of inertia.
→ An object with more moment of inertia is harder to stop once it’s rotating.
→ An object with less moment of inertia is easier to stop or change direction.
🚗 Inertia – Car Example– When you’re sitting in a car and the driver suddenly hits the brakes, your body lurches forward.
Why?
Because of inertia, your body wants to keep moving at the same speed the car was moving earlier. Now the car is slowing down and stopping. However, your body resists this change and continues moving forward.
So, the angular momentum basically describes the tendency of an object to continue rotating around a particular axis. Check this video on Angular momentum – https://youtu.be/ho3VQy4wO4E?si=g9Be9epJsoQ9Jhpp
Some sub-atomic particles, like electrons, have intrinsic angular momentum, though they are not moving in an angular way. This is because these particles behave in a certain way in magnetic fields. This is referred to as the ‘spin‘ of that particle. Note that, when these particles actually rotate or spin, the total angular momentum of these particles will be the sum of –
Intrinsic angular momentum + angular momentum due to actual motion
In a quantum mechanical context –
Electrons in orbitals with higher angular momentum quantum numbers (ℓ) have:
→ More complex orbital shapes (e.g., s < p < d < f)
→ Greater probability density farther from the nucleus
→ Less tightly bound to the nucleus
Electrons with lower angular momentum (e.g., s-orbital, ℓ = 0):
→ Can penetrate inner shells more effectively
→ Are more localised near the nucleus
→ Show stronger nuclear attraction.
How do we measure the spin?
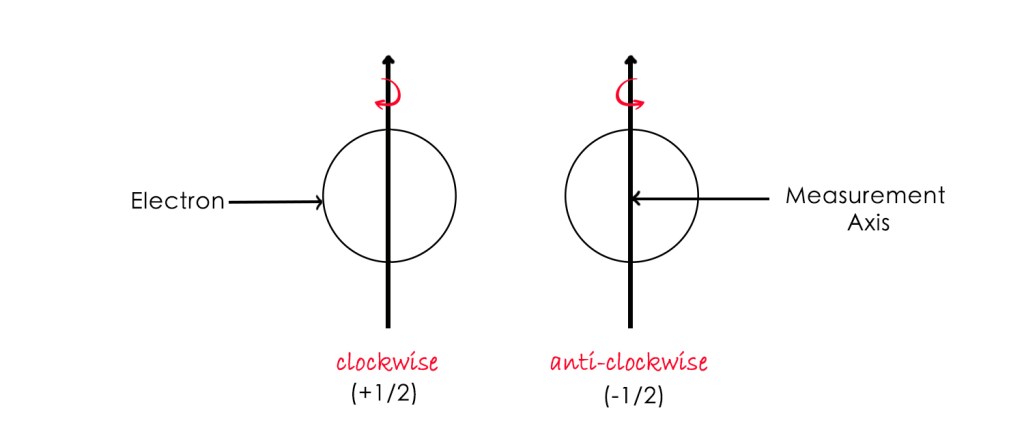
First, the direction for measuring the spin is determined. The axis, having a particular direction, which we choose, is called the measurement axis. For an electron, there are two possibilities –
- The spin of the electron aligns with the measurement axis – spin up – (+1/2) value
- The spin of the electron is in the opposite direction – spin down – (-1/2 ) value
These are the two different states of the electron spin. When the electron is behaving like it’s moving clockwise and anti-clockwise directions (w.r.t measurement axis), it is in the ‘spin up’ and ‘spin down’ states, respectively.
Spin is quantised
In quantum mechanics, the electron spin is a quantised intrinsic property of electrons, meaning it can only take on specific, discrete values. Thus, spin magnitude is not variable; it takes on only one specific value.. It can either be +1/2 or -1/2.
In a general sense, this means that the electron can spin only at a certain speed- it can’t go slower or faster than that.
Now, with the thorough understanding of electron spins, we shall proceed to discuss an important concept in photochemistry in the next post.
Till then,
Be a perpetual student of life and keep learning…
Good day!