As we saw in the earlier post, though the Bohr model was successful in paving the way into the structure of the atom, it was not a full-proof model. One of the most revered physicists of that time Albert Einstein hated Neils Bohr’s idea of the atom.

Though Bohr and Einstein shared a mutual admiration for each other, there was a lack of agreement between the two, over the concept of Quantum Mechanics. There were innumerable public disputes between the two physicists. Niels Bohr was supporting the quantum mechanical theory and Einstein totally rejected it. However, the limitations of the Bohr model were hinting that there was more mystery hidden in the atom. Scientists all over the world started delving deeper into this arena of science and atomic physics became the hot topic of research. Everyone wanted to know more about the atom! This infinitesimal part of the matter was holding so many secrets in it which the scientists of that era were trying to unravel.
We have seen in the earlier post that the Bohr model of the atom was unable to explain the doublet of lines and splitting of spectral lines. Let us first understand what were these phenomena.
A]Doublet of lines –

In 1887, two scientists, Michelson and Morley studied Angstrom’s spectral lines (3→2 lines)more closely with better equipment and found that an individual line was, in fact, a set of two very closely spaced lines i.e it was a doublet.
If a doublet is observed it clearly indicates that there were two energy levels closely spaced to each other. So, maybe there was some detail in the structure of the orbits which the Bohr Model did not encompass. Bohr’s model could not explain the occurrence of this doublet.
B]Splitting in magnetic field –
In 1896, a Dutch scientist, Zeeman, was studying the effect of magnetic field on the spectral lines of the emission spectra of substances. He placed the gas discharge tube in a magnetic field and studied the emission spectra. He observed that in a steady applied magnetic field, the spectral lines split into doublets or triplets. This phenomenon is called ‘Line Splitting’.
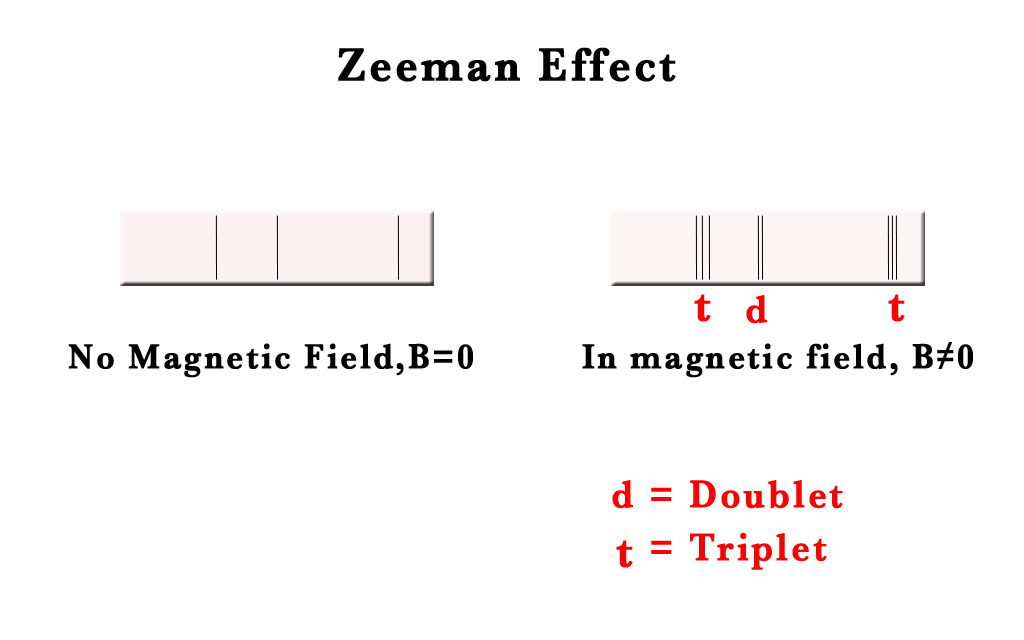
The intensity of the magnetic field ∝ degree of Splitting of lines.
So, the higher the magnetic field he applied, the more spread out the split lines were. Zeeman won a Noble prize in 1902.
C]Splitting in electric field –
In November 1913, just after Bohr’s paper was published (June 1913), a German scientist, Starck, was studying the effect of electric current on the spectral lines and he observed a similar effect to Zeeman. Under the influence of an external electric field, the spectral lines split into doublet or triplets. The intensity of the splitting was directly proportional to the intensity of the applied electric current. These phenomena were pointing to some hyperfine structure in the atom and the Bohr model made no reference to these.

In 1916, a theoretical physicist in Munich – Germany, Arnold Sommerfeld helped to visualize the atom in a slightly different way, which would explain the doublets and triplets, which were observed experimentally. He proposed some modifications to the Bohr model. This great physicist has a record of being nominated 84 times(max number of times in history) for the Nobel Prize but he never received it! However, his students, Werner Heisenberg, Wolfgang Pauli, Peter Debye, Linus Pauling, and Hans Bethe went on to receive their respective Nobel prizes!! Many of his students made a mark for themselves under his guidance. He was a great mentor to his students.Albert Einstein told Sommerfeld:
“What I especially admire about you is that you have, as it were, pounded out of the soil such a large number of young talents.”
THE SHELL MODEL –
Sommerfeld replaced Bohr’s circular orbits with elliptical orbitals or shells and also introduced azimuthal and magnetic quantum numbers. He stated that the orbitals were like the egg shells (having some thickness) and so his model is also known as the ‘SHELL MODEL’.
He suggested that in addition to a circular orbit, the electron also moves in one or more elliptical orbitals and these orbitals are roughly the same size and are of similar energies as compared to the circular orbit. He thus put forth an idea where, there could be some fine structure associated with every orbit which is described by the principal quantum number, n, proposed by Bohr. All these closely spaced orbits (circular) and orbitals (elliptical ones) form a band-like/ shell-like structure. Thus, the name the ‘Shell model’.
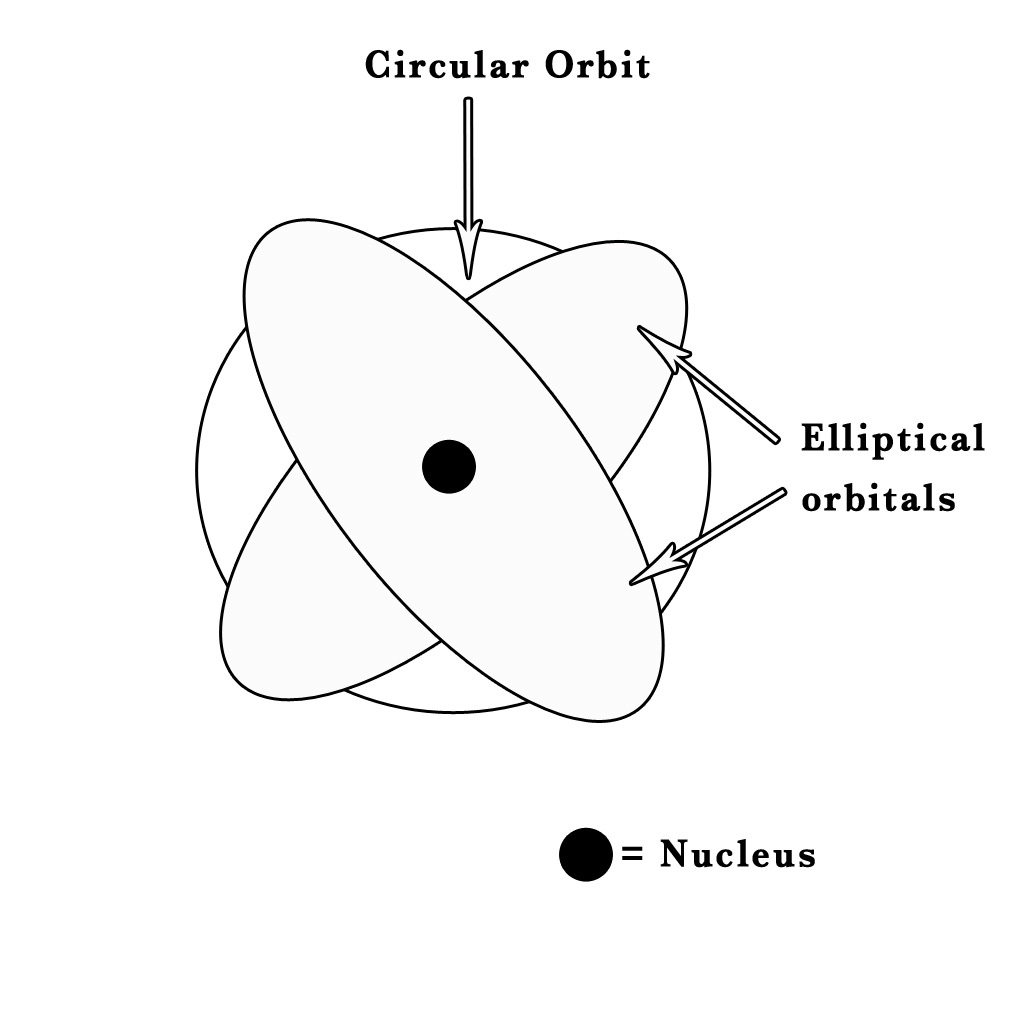
The Shell can be quantitatively represented as the quantum number ‘n’ – the principal quantum number. However, to describe the sub-shells Sommerfeld put forth the idea of a new quantum number – the Orbital/Azimuthal quantum number(ℓ). Each shell is subdivided into sub-shells called s,p,d,f. He further introduced the magnetic quantum number(m) to define the orientation of the sub-shells in space. Thus, the three quantum numbers required to describe the electron in an atom are as follows –
- Principal quantum number (n) – It gives us the SIZE of the orbit or its distance from the nucleus.n= 1,2,3…
- Azimuthal /Orbital quantum number (ℓ)– It tells us about the SHAPE of the orbital.
ℓ= 0,1,2,3…..,(n-1).e.g. ℓ = 0 defines the s-subshell. - Magnetic Quantum number(m)– This describes the ORIENTATION of the orbitals in space.m= ℓ,(ℓ-1) , …0…-ℓ.
The Stern–Gerlach experiment –
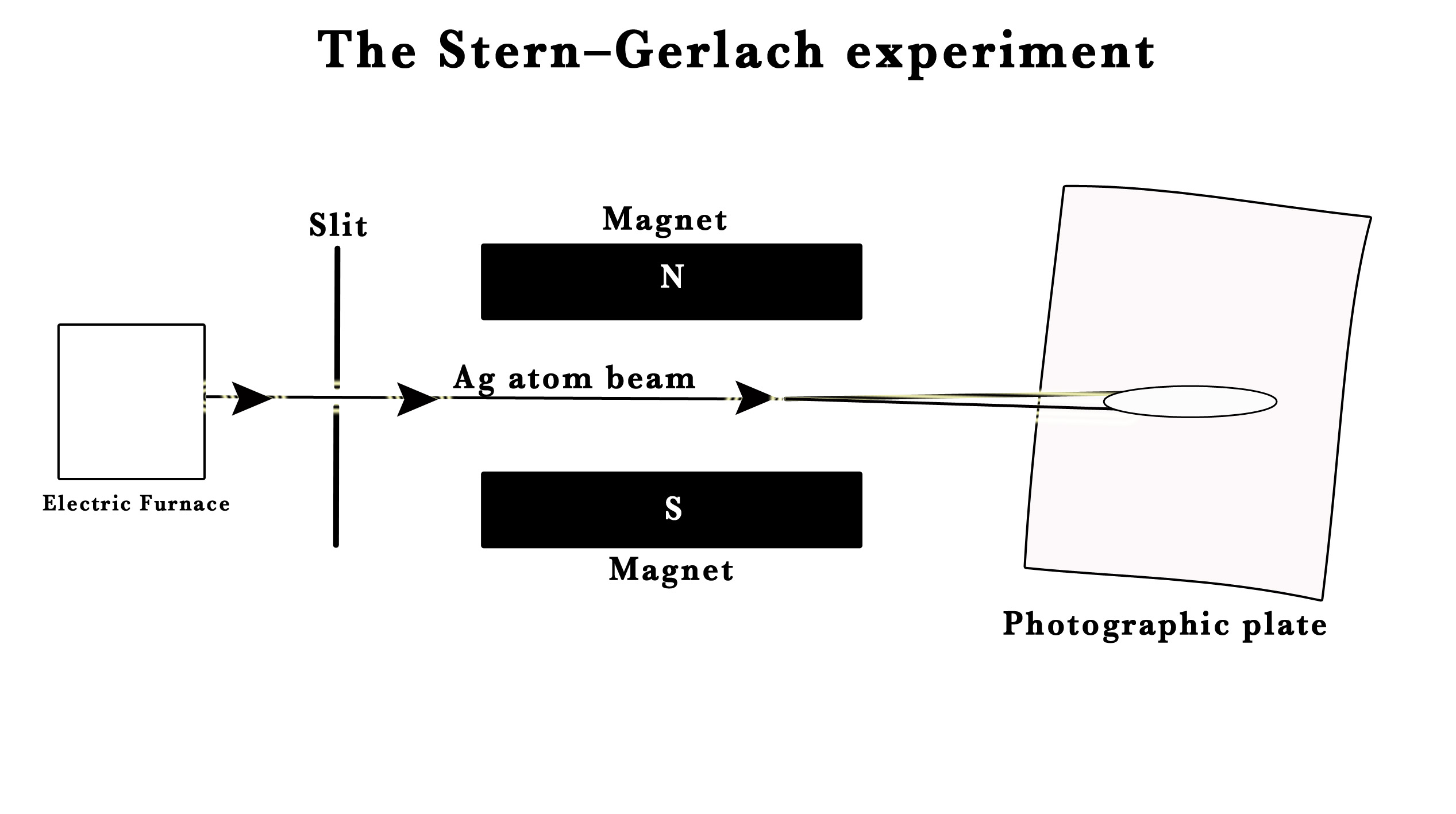
In 1921, Otto Stern and Walter Gerlach, conducted an experiment to study the magnetic properties of a single electron.
They choose Silver metal to conduct the experiment because it contains a single electron in its outermost shell,
Electronic Configuration of Silver (47) → [Kr] 4d10 5s1.
This single electron is shielded from the nucleus by the inner 46 electrons. Since this valence electron is in the s- subshell, its angular momentum is zero (ℓ = 0) and so technically it would not interact with any external magnetic field. Also, silver is easily detectable on a photographic plate.
Apparatus –
- Electric furnace was used to produce a beam of silver atoms.
- A slit to focus a single beam onto the screen.
- Photographic plate used as a screen to study the impact of silver atoms on it.
- Non uniform magnets to create a non uniform magnetic field.
They shot a beam of silver atoms through a slit and passed this beam through a non-uniform magnetic field. They expected to get a continuous smear on the photographic plate, like a shadow of the slit, indicating that the beam passed undeflected through the magnetic field. Instead, they saw that the field split the beam into two separate parts!
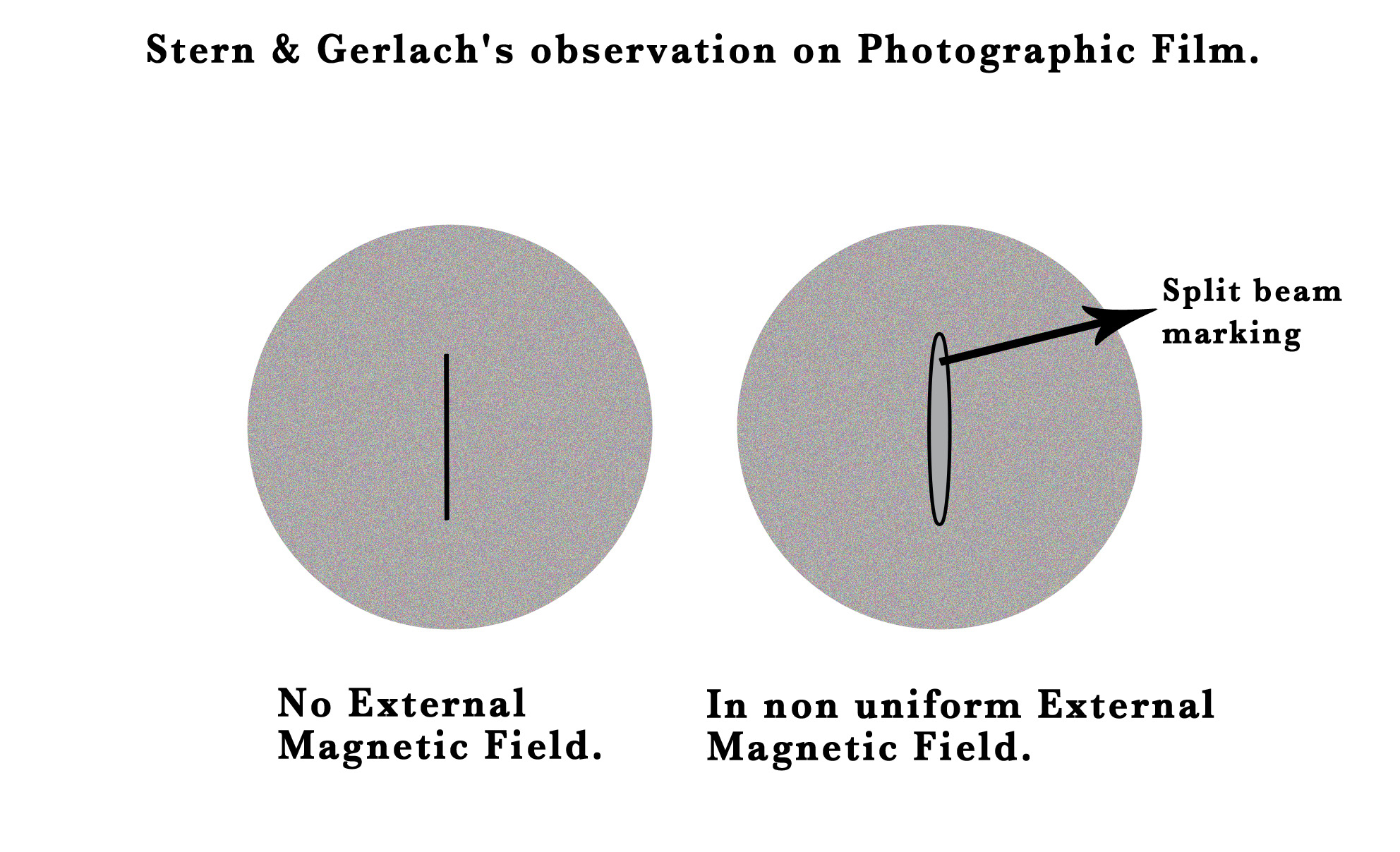
If the valence electron of silver had no angular momentum and the external magnetic field had no effect on it, then why was it that the beam split into two? The only plausible reason for this observation was that the electron had two different orientations of the magnetic moment.How was a magnetic moment produced without the angular momentum?
(The angular momentum and magnetic moment are closely related to each other. I shall not get into the details of this, as it is not under our course of study. If someone wishes to get into the details of this relation, kindly search for the two and gyromagnetic ratio concepts on Google).
Even after making these observations, Otto Stern and Walter Gerlach, could not conceive the idea of electron spins themselves. It was in 1925, that Samuel A. Goudsmit and George E. Uhlenbeck conceptualized the idea of electron spins! They were graduate students and they proposed that the electron doesn’t just revolve around the nucleus but also spins around itself!! On this occasion, Goudsmit quoted,
“The Pauli principle was published early in 1925… if I had been a good physicist, then I would have noticed already in May 1925 that this implied that the electron possessed spin. But I was not a good physicist and thus I did not realize this… Then Uhlenbeck appears on the scene… he asked all those questions I had never asked… When the day came that I had to tell Uhlenbeck about the Pauli principle – of course using my own quantum numbers – then he said to me: “But don’t you see what this implies? It means that there is a fourth degree of freedom for the electron. It means that the electron has a spin, that it rotates”… I asked him: “What is a degree of freedom?” In any case, when he made his remark, it was luck that I knew all these things about the spectra, and I said: “That fits precisely in our hydrogen scheme which we wrote about four weeks ago. If one now allows the electron to be magnetic with the appropriate magnetic moment, then one can understand all those complicated Zeeman-effects.”
– S. Goudsmit
They explained the observations made by Stern and Gerlach, stating that the valence electron in the silver atom is unpaired. The spins of the other 46 electrons cancel each other but the last valence electron’s spins are manifesting themselves in these experimental results. This last electron can have two spin orientations according to the right-hand rule- spin up↑ and spin down↓ . So, half of the silver atoms have spin-up valence electrons and half of them have spin-down valence electrons and thus, the beam splits into two in an external magnetic field, revealing the two orientations of the electron spins! The energy of an electron would depend upon whether its angular momentum due to spin is aligned or anti-aligned with the angular momentum due to its rotation about the nucleus. This was responsible for the slightly different spectral frequencies observed. This was the birth of the fourth quantum number – THE SPIN QUANTUM NUMBER(s)! s= +1/2 or -1/2.
So, what happened with the advent of these four quantum numbers? What do these quantum numbers mean? What is the significance of these 4 quantities? Was the Sommerfeld-Bohr model a flawless atomic model? We shall discuss all this in our next post. Till then,
Be a perpetual student of life and keep learning..
Good day!
References and Further Reading-
1)https://en.wikipedia.org/wiki/Arnold_Sommerfeld
2)Lecture 05 The Shell Model Bohr Sommerfeld Model and Multi electron Atoms; Quantum Numbers n, l, m by Professor Sadoway.
Image Source –
1)By Paul EhrenfestOriginal uploader was Graf at de.wikipedia – http://www.dfi.dk/dfi/pressroom/kbhfortolkningen/, Public Domain, https://commons.wikimedia.org/w/index.php?curid=141589
2)https://upload.wikimedia.org/wikipedia/commons/d/d5/Niels_Bohr_Albert_Einstein_by_Ehrenfest.jpg
One comment