This post is dedicated to a scientist who gave us the modern periodic table – Henry Moseley!

Henry Moseley was born into a family of science scholars.Both his grandfathers were fellows of Royal society and his father was a professor of biology at the Oxford university.He earned a bachelor’s degree from Trinity college and later went to Manchester to work with Ernest Rutherford.
He found his initial days of working in Manchester ‘not too exciting’ but still continued his work with the radioactivity project.In 1912 his experimental equipment broke and that is when he steered his energies into a new research – X rays! Though his mentor Rutherford was not conducting reasearch on X-rays, he along with Charles Darwin(the grandson of the Darwin), convinced Rutherford that they could work on this topic .
They both started conducting research without knowing what would come out of it.Darwin quoted,
“Working with Moseley was a most impressive experience. He was without exception the hardest worker I have ever met. He had two principles in his work. The first was that when one starts to set up an experiment one must not stop for anything until’it is set up. The second was that when one starts the experiment itself one must not stop till it is finished. There were of course no regular meals, and work often went on for most of the night. Indeed one of Moseley’s expertise was the knowledge of where one could get a meal in Manchester at 3 o’clock in the morning.”
Later Darwin left this research to work on a different project .Moseley was determined, ready to go ahead all by himself and study X-ray patterns of elements. He placed different elements on a railway, from Calcium to Zinc, in a X-ray tube and bombarded them with electrons, one by one. The elements gave off X-rays , which passed through a prism to produce a X-ray spectrum.
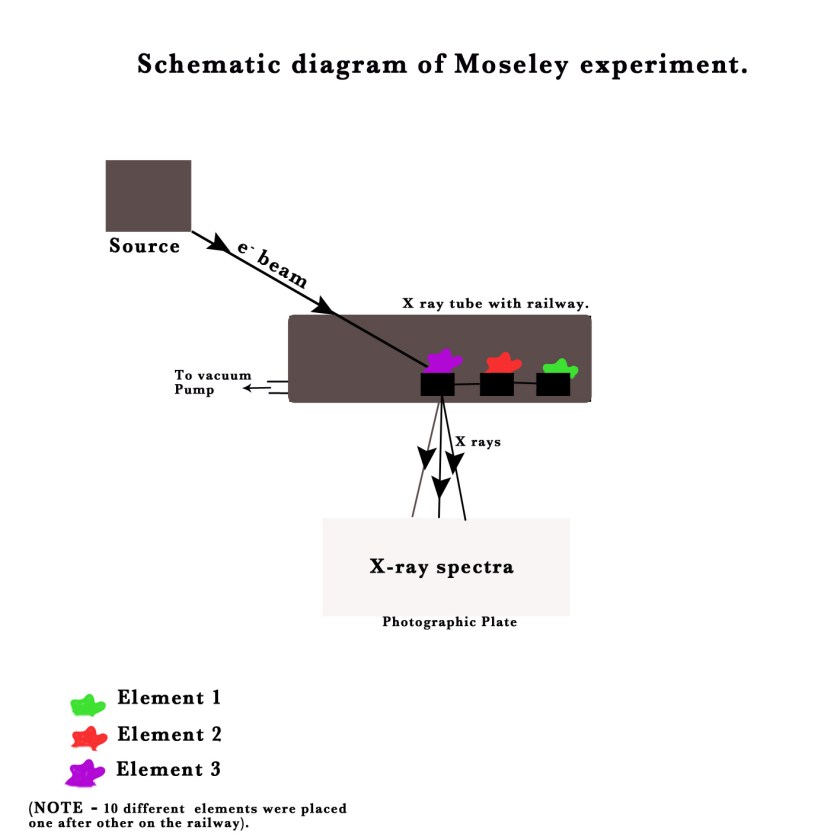
He found that each element gave a unique X-ray spectrum characteristic of that element. It was like the element’s fingerprint! He later arranged the various spectra of adjacent elements according to their frequencies and found that there was a simple pattern to them! The dominant frequency lines rose step by step like a ladder. His spectras revealed that Cobalt and Nickel were placed correctly in the periodic table, even though their atomic weights dictated otherwise. The X-ray spectrum was dependent on the atomic number(Z) of an element – the number of protons in the nucleus of the element. Thus, he concluded that the properties of elements were not dependent on their atomic weights but on their atomic numbers! He thus, put the Mendeleev periodic table in new light and gave us the modern periodic law –
The properties of the elements are periodic functions of their atomic numbers.
Moseley gave us the Modern periodic table! He also precisely predicted that only 7 elements were yet to be discovered. During 1914, a war broke out and England was a part of the war. Moseley was determined to do his duty towards the country and so he volunteered to join the army. Ernest Rutherford advised him not to do so as he didn’t want Moseley’s amazing work to stop. Moseley was undoubtedly one of the brightest students Rutherford was mentoring, but Moseley did not pay heed to Rutherford’s request.
Moseley was not easily admitted in the army as he was basically an engineer ,but he pestered the authorities to let him serve his country.In 1915,when he was just 27 years old, he was commissioned to go to Turkey as a communications officer.During the war, he was shot in the head by Turkish troops and killed .This was a terrible blow to the scientific community especially to Ernest Rutherford , who thought that this was one of the greatest loss during the WORLD WAR I.
By the end of 1945, every element Moseley had predicted was discovered.The seven elements that were found were – Protactinium (1917), Hafnium (1923), Rhenium (1925), Technetium (1937), Francium (1939), Astatine (1940), and Promethium (1945).In the mean time, the next generation of scientists had learned to make new synthetic elements.Thus, these new elements also found their place in the table and thus was formed – THE MODERN PERIODIC TABLE.

The periodic table is a grid in which the elements are arranged in order of increasing atomic numbers.The periodic table arranges all the elements, with such diverse properties in order.A well defined pattern is thus seen in the arrangement of elements.This pattern is also referred to as ‘periodicity’.
In the above table –
The rows are called PERIODS .There are 7 periods.The properties change gradually and systematically across a period.The elements to the left and right of the periodic table show very different properties.
e.g. – Consider the third period. Sodium (Na) which is to the extreme left of this period, is a metal,a solid and a very good reducing agent, due to its tendency of loosing its outermost electron(Sodium after loosing its outermost electron, achieves octet stability from its penultimate shell).Whereas Chlorine ,which is to the far right (before Argon) is a non-metal,a gas and a very good oxidizing agent pertaining to its tendency of accepting an electron to complete its octet.(Chlorine has 7 electrons in its outermost orbit and by accepting one electron from a donor it gets its octet stability – Stability on account of completely filled shell).
The 7 periods of elements are as follows :-
- First period has only 2 elements H and He and thus it is called very short period.
- Second period has 8 elements Li to Ne called short period.
- Third period has 8 elements Na to Ar called short period.
- Fourth period has 18 elements K to Kr called long period.
- Fifth period has 18 elements Rb to Xe called long period.
- Sixth period has 32 elements Cs to Rn called very long period.14 elements (Z=57 to Z=71) are placed separately at the bottom of the periodic table.They are called Lanthanides,
- Seventh period has 28 elements from Fr to Uuq and is called incomplete period.Another 14 elements (Z=89 to Z=103) are also separately placed at the bottom of the periodic table.They are called Actinides.
The columns are called GROUPS.There are 18 groups,which are divided into 9 main groups –
I, II, III, IV, V, VI, VII, VIII and 0 groups.
The groups I to VII has two sub groups each called A and B.Group VIII B has 3 rows of elements.
0 group has one row of elements.
|
A sub group |
Normal elements |
|
B sub group |
Transition elements |
|
IA group |
Alkali metals |
|
II A group |
Alkali earth metals |
|
VII A group |
Halogens |
|
O group |
Noble gases |
|
Lanthanides & Actinides |
Inner transition elements |
Elements in a group have similar properties owing to similar outer electronic configuration.The number of valence electrons is the same for all elements in a group.
e.g. – the outer electronic configuration of the halogen group(Group 17) is ns2 np5.
|
Halogens |
Atomic number |
Electronic Configuration |
|
Fluorine |
9 |
1s2 , 2s2, 2p5 |
|
Chlorine |
17 |
1s2 , 2s2, 2p6, 3s2 , 3p5 |
|
Bromine |
35 |
1s2 , 2s2, 2p4, 3s2 , 3p6, 3d10, 4s2 , 4p5 |
|
Iodine |
53 |
1s2 , 2s2, 2p4, 3s2 , 3p6, 3d10, 4s2 , 4p6,4d10,5s2,5p5 |
|
Astatine |
85 |
1s2 , 2s2, 2p4, 3s2 , 3p6, 3d10, 4s2 ,4p6, 4d10, 5s2, 5p6, 5d10, 6s2, 6p5 |
Natural elements –
There are total 118 elements in the periodic table of which elements from Z=1 to Z=92 are naturally occurring elements(except Technetium, Tc Z= 43 and Promethium, Pm Z= 61).
Transuranic Elements–
All elements after Uranium are artificially synthesized and are called transuranic elements. So far 26 elements are made artificially from Z=93 to Z=118.Most of these elements are radioactive and each of them decays into other lighter elements.
Chemical symbols.
Each element in the table has its own chemical symbol. e.g.– C for Carbon, Au for gold , S for sulphur etc. All chemical symbols start with capital letters and if there are two or more letters then the only the first letter is a upper case. The following letter/s is/are lower case. e.g. Sodium Na , Chlorine Cl , Rhodium Rh , Ununpentium Uup.
Some elements have been named after –
- scientists e.g.– Rutherfordium Rf , Bohrium Bh.
- city where the element was discovered e.g.- Berkelium Bk, Californium Cf .
The symbols of elements like gold and silver are Au and Ag respectively as these represent their Latin names Aurum and Argentum.
We shall talk more about the long form of periodic in our successive posts.
Till then,
Be a perpetual student of life and keep learning..
Good day !
References and further reading –
1)https://en.wikipedia.org/wiki/Henry_Moseley
2)http://blog.oup.com/2013/04/henry-moseley-elements-x-ray-periodic-table/
3)THE MYSTERY OF MATTER:Into the Atom documentary.
4)http://www.iucr.org/__data/assets/pdf_file/0018/765/darwin.pdf
5)https://en.wikipedia.org/wiki/Synthetic_element
6)https://docs.google.com/presentation/d/1Hw3G0Q11SIXWx9_PFZmMQq5jKLRSlzYUpv_I9gqa_LY/edit#slide=id.p22
Image Sources-
1.https://en.wikipedia.org/wiki/Henry_Moseley#/media/File:Henry_Moseley.jpg
2.https://sciencenotes.org/periodic-table-wallpapers/