In our last post we introduced the modern form or long form of the periodic.Let us now begin to study the table in greater detail.
We studied that the periodic table of elements contain elements.But what are elements?
Elements are primary constituents of matter which cannot be divided further into other substances by any physical or chemical process.
e.g.- Copper,Aluminium,Gold,Silver etc.
This is unlike compounds which can be converted to the parent elements by chemical process.
e.g.- Table salt i.e NaCl can be converted to Sodium (Na) and Chlorine(Cl) by electrolytic process. Sodium and Chlorine are elements.

Elements can be classified according to their state(at room temperature and normal pressure)-
- More than 100 elements are in solid state.
- Eleven are in gaseous state.
- Two in liquid state – Bromine and mercury. However, room temperature is a vague term. In scientific terms, room temperature is considered either 20ºC or 25ºC.However, when the temperature is slightly warmer (≈26-40ºC) the following four elements exist in liquid state too –
Francium, Cesium, Gallium, Rubidium.
Further, the elements in the periodic table can be broadly classified into 3 main categories namely – METALS , NON – METALS and metalloids.Majority of the periodic table is occupied by metals.There are a few metalloids and the rest are non-metals.

METALS .

Metals are elements which are generally solid, which form positive ions by loosing electrons in a chemical reaction and wherein the atoms are bonded to each other via metallic bonds.The word ‘metal”is derived from Greek word ‘metallon‘ meaning ‘to mine‘ or ‘excavate from the ground’.
Let us first discuss the nature of metallic bonds.
The different atoms in a metal are held together by a force known as metallic bond.

As you can see in the adjacent figure,the outer/valence electrons of a metal are loosely bound to the nucleus and are thus free to move around in the metal lattice.These electrons are shared by all atoms.This collection of electrons is also called as ‘sea of delocalised electrons’.The metallic bond is the electrostatic force of attraction between these valence electrons and the nuclei of atoms.This structure is responsible for majority of the properties of metals which distinguish them from the other elements in the periodic table.
Properties of Metals –
1)Metals have comparatively less number of valence electrons and so they tend to be electron donors.Thus,generally they have a tendency to form positive ions.
e.g
1. Na → Na+ + e –.
2.Al → Al3+ + 3e–.
2)Metals have lustre i.e they are shiny.The word ‘LUX’ means ‘light/radiance’ in Latin.Just step into a jeweller store and we know how gold and silver surfaces shine.Copper vessels have a shiny surface too.
The free electrons in a metal crystal are responsible for this shine.The incident light causes vibrations in the free electrons which in turn produce their own light.We see this as the lustre.Some metals lose their lustre on account of their interaction with air,water or carbon.Thus, they form compounds (e.g. – oxides) and get tarnished or rusted.Whern this rust is removed, we get the shiny metal surface back. Watch the following video to see how that happens –
The rusted surface doesn’t shine as the free electrons react and are no longer free to reflect the incident light.
3)Metals are malleable i.e they can be drawn into sheets.We have already studied Ernest Rutherford’s Gold foil experiment.The thin gold foil could be made as a result of this property of metals.We even use Aluminium foil in our kitchen on a daily basis.Metal sheets/foils can be created as they have the ability to withstand the pressure during the hammering process.
So a metal doesn’t break but bends.I am sure you have noticed a dent on the body of the car(made of metal) after it got hit.The metal body bends due to the impact but hardly breaks.
Metals are malleable as the layers of atoms in a metallic bond can slide over each other,when the metal is bent.This is possible because the metallic bond is nondirectional -same in all directions.

4)Metals are ductile i.e they can deform (but not break) under tensile stress and can be drawn into wires.When streched a susbstance which is not ductile breaks.A ductile substance doesnot.
(Note- Tensile stress/force is opposite of compressional force.It is the force that pulls apart along the axis just like people pulling a string in tug-of -war.In the figure below, tensile strength is shown by turquoise arrows).

This property of metals is also a result of their structure.As the layers of atoms slide over each other without breaking, metals can be easily drawn into wires by applying tensile stress.This property is very useful as metal wires are used to conduct electricity.Mostly all the electric appliances ,electric wiring in our homes are made of metal wires.These wires are have a PVC (polyvinyl chloride ) coating to prevent the user from getting electrocuted.
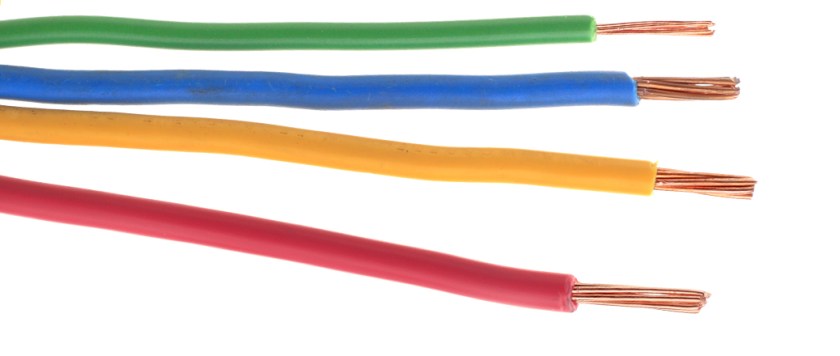
5)All metals are good conductors of heat and electricity .The free, delocalized electrons in a metal crystal help in conducting heat and electricity efficiently.As these electrons can move freely through the metal structure, electricity and heat can also flow through metals effortlessly.
 When a metal is connected to a cell/battery, the electrons get repelled by the negative terminal and start flowing towards the positive terminal of the cell/battery.This generates an electric current.Silver is the best conductor of electricity i.e it has highest electrical conductivity.The next best conductor is copper and most of the conducting wires are made of copper as it is much economical than silver.Metals like Iron and mercury offer more resistance to the flow of electric current through them and thus have lower electrical conductivity.
When a metal is connected to a cell/battery, the electrons get repelled by the negative terminal and start flowing towards the positive terminal of the cell/battery.This generates an electric current.Silver is the best conductor of electricity i.e it has highest electrical conductivity.The next best conductor is copper and most of the conducting wires are made of copper as it is much economical than silver.Metals like Iron and mercury offer more resistance to the flow of electric current through them and thus have lower electrical conductivity.

Similarly,when heat is transferred to a metal at one end, the heat causes the free electrons to vibrate rapidly and thus, their kinetic energy increases.The electrons transfer the vibrational energy to the colder end, thus conducting heat in the process.
6)Metals have high melting and boiling points compared the non-metals in the same row.This property can be ascribed to metals on account of the high strength of metallic bonding.The strong force of electrostatic attraction between the positive kernels and the free negative electrons makes the metallic bond quite strong and thus to break it high amount of energy is required.
|
Metal |
Boiling point |
Non Metal |
Boiling Point |
|
Sodium |
882.8 °C |
Chlorine |
-34.04 °C |
|
Potassium |
758.8 °C |
Bromine |
58.8 °C |
|
Bismuth |
1564 °C |
Astatine |
336.8 °C |
|
Metal |
Melting point |
Non Metal |
Melting Point |
|
Sodium |
97.79 °C |
Chlorine |
-101.5 °C |
|
Potassium |
63.5°C |
Bromine |
-7.2°C |
|
Barium |
727 °C |
Astatine |
301.8 °C |
Amongst metals,Gallium,Caesium ,Sodium and Potassium have low melting points -30°C ,28°C, 98°C and 64°C respectively.Gallium and caesium strt to melt in hands due to our body temperature.
7)Metals are sonorous i.e they produce a deep sound when struck.It is due to this property that metals are used to make bells .The bells in India are made up of a mixture of five metals(Panchdhatu) – lead, copper, zinc, iron, and tin in specific proportions , which create a reveberating sound,whose echo lasts for 7 seconds ,enough to touch the seven chakras in our body.

When a metal is hit,the free electron cloud gets disturbed and the kinetic energy gets easily propagated through them as a wave on account of their delocalised nature.The sound is produced as the metallic bond is elastic in nature. The electrons swing due to the hit and this swinging motion is passed to the air around the metal.The swinging motion of the air causes the sound.
8)All metals except mercury (which is a liquid) are solids at room temperature.They are hard, have high densities.Although sodium and potassium are soft metals and can be cut with a knife. I am sure, most of you have handled sodium in your Chemistry laboratories- picked a piece of sodium ,which is kept inside kerosene and cut it with a knife.
9)Metals form basic oxides unlike nonmetals.
e.g.- Sodium forms sodium hydroxide (NaOH) in water which is basic in nature.Metal oxides generally form hydroxides in water and produce OH– ions , which make them basic in nature.
Na2O + H2O → 2NaOH
The NaOH will dissociate in water to produce OH– ions.
Based on their position in the periodic table, metals can be categorised as –
- Alkali metals – Group I
- Alkaline earth metals – Group II
- Transition metals
- Lanthanides/Rare earth metals
- Actinides.
So,now we have a fair idea of what metals are and their proeprties.In our next post we shall talk about the non-metals and metalloids.Till then,
Be a perpetual student of life and keep learning..
Good day!
References and further reading –
1)http://oureverydaylife.com/role-electrons-play-metals-luster-34550.html
2)https://en.wikipedia.org/wiki/Ductility
3)https://www.reference.com/science/metals-good-conductors-heat-electricity-5ddc9d18fc1508d2
6)Science for tenth class part2 Chemistry by Lakhmir Singh and Manjit Kaur.
7)http://physics.stackexchange.com/questions/231547/why-does-metal-make-sound-when-it-is-hit
Image Sources-
1)https://www.angelo.edu/faculty/kboudrea/periodic/physical_metals.htm
2)http://joelynchelectrical.com/project/wiring-and-re-wiring/
3)https://www.emaze.com/@AIRLCFQO/Iron-Element-Project
4)http://www.lipglossiping.com/wp-content/uploads/2013/02/gold-nugget.jpg
5)http://www.wisegeek.com/what-is-platinum.htm
6)http://www.indiadivine.org/science-behind-bells-hindu-temples/

