We shall begin our discussion on the general periodic trends of the periodic table from this post onwards.Periodicity means the tendency to recur/repeat at regular intervals.The table of elements manifests this phenomenon in many ways. The arrangement of elements is such that their properties show a periodic trend across a row and down a group.Let us start studying the various properties of elements , which gives the arrangement of elements in the periodic table a beautiful pattern.
Atomic Number (Z)–
Atomic number (Z) is the number of protons found in the nucleus of an element.
It can also be defined as the number of electrons in a neutral atom.
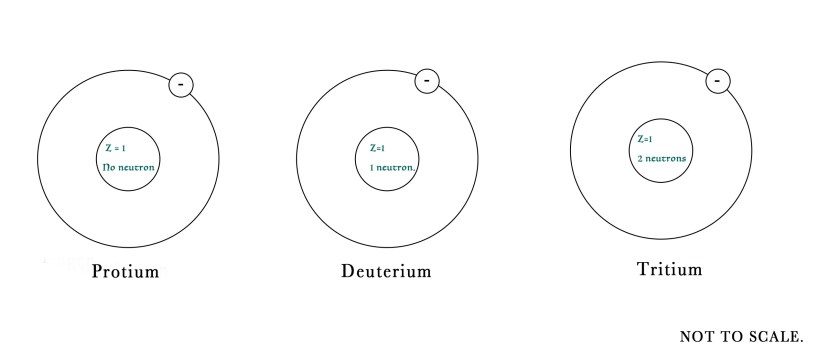
e.g.– Atomic number of hydrogen is Z = 1, as it contains only 1 proton.Deuterium, tritium which are isotopes of hydrogen also have the same atomic number as the number of protons in their nucleus remains the same (although number of neutrons is different).
We have already studied that ‘the properties of elements are periodic functions of their atomic numbers’. The elements are arranged in increasing order of their atomic numbers in the periodic table. Atomic number is the most fundamental property, as it dictates the properties an element possess. Atomic number gives an element its chemical identity.
Atomic Mass Number (A)–
This quantity gives us the number of protons and neutrons in the nucleus of an atom.As the atomic number increases with each element in the periodic table , so does the atomic mass number. As seen above the three different isotopes of hydrogen have the same atomic number but their atomic mass number distinguishes them from one another.
The atomic number(Z) is written as a subscript and atomic mass number(A) is written as superscript, to the left of the element symbol.

(NOTE – How to remember this? Alphabetically A is always at the top and Z at the bottom.Similarly, the mass number(A) is written at the top and the atomic number (Z) at the bottom).
So, we can conclude that isotopes of an element have the same atomic number but different atomic mass number. e.g.- 35Cl and 37Cl are isotopes of chlorine having atomic mass numbers 35 and 37 respectively.
A=Z + n ,where
A= Atomic mass number
Z=Atomic number
n= no.of neutrons in the nucleus.

Atomic Size/radii –
- In general ,atomic radius is the distance from the nucleus to the outermost occupied shell of the atom. Whether one measures the distance from the centre of the nucleus or from the circumference is immaterial , as the size of the nucleus is negligibly small compared to the size of the atom.
Size of the atom =10-10m i.e 10000000000 atoms would approximately fit in 1 meter distance.
Size of the nucleus = 10-15m.
So, the nucleus is 1/100000 th times smaller than the atom. So the nucleus is like a pea in the middle of the racetrack or a spec of dust in a very large room ! - Also , we know that orbitals are nothing but spaces where the probability of finding electrons is the highest.So, we have to consider an arbitrary edge for the valence atomic orbitals.
- Atomic radii increases down a group as every time a new valence shell is added around the nucleus as a result of increasing atomic number.
e.g. – Group 1 Li,Na,K ,Rb,Cs,Fr atomic size increases down the group (See figure below). - Atomic radii decreases from going from left to right across a period as –
- electrons are added to the same shell.
- electrostatic force of attraction between protons in the nucleus and electrons increases as atomic number increases.
Across a period, atomic number increases ⇢ # of protons increases ⇢ # of electrons increases ⇢ the electrostatic force between them also increases.
It’s as if the love/attraction(electrostatic) between protons and electrons increases and thus they all keep coming closer together, resulting in decrease in atomic radii.

- In Chemistry, there are different types of radii we consider according to the type of species under study –
Van der Waals radii
Ionic radii
Covalent radii
Bohr radii
Metallic radii
We shall discuss about these, in detail , in our later posts. However, in this post , let us just learn the difference between Van der Waals and covalent radii , as this will help us to understand, why the size of noble gases is more than the halogens in the same period(see the figure above).
Covalent radius ⇒ half the distance between the nuclei of two atoms bonded together covalently. e.g.- Cl2, F2 , N2 , O2 etc
Van der Waals radii ⇒ half the minimum distance between the nuclei of two atoms of the element that are not bound .e.g.- Noble gases like Ar,Xe,Kr etc.

- Noble gases(who are expected to have smallest radii) have greater size than halogens in their respective period because –
- In Noble gases, the octet is complete in the valence shell.Thus, there is maximum repulsion between their electrons.This repulsion leads to increase in size.
- We measure Van der Waals radius for Noble gases and covalent radius for other diatomic elements, e.g. – Halogens.
- Atomic radii trends in transition metals are slightly different as in their case, the additional electrons are added in penultimate d-orbital. The general electronic configuration of d block/transition elements is – (n-1)d1–10 ns1–2. In this case, there is no change in the valence ns shell/orbital. The electrons keep adding to the (n-1) d orbital, which is penultimate orbital.
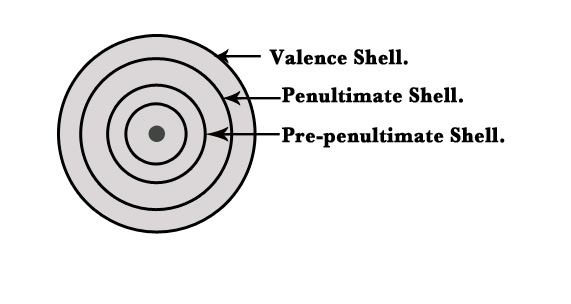
FYI –
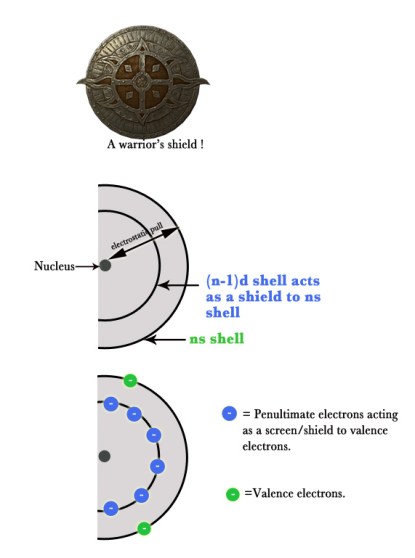
There are two contrasting effects, which balance one another, in transition elements –
♦As the atomic number increases across a period, the electrostatic pull between the protons in the nucleus and the valence electrons increases.This results in decrease in the atomic size.
♦Although, as the atomic number increases, the electrons get added to the penultimate shell.These penultimate electrons start shielding(protecting) the valence electrons from the nuclear pull.Thus, the valence electrons experience lesser electrostatic pull.So, the size increases.
e.g. Scandium has one 3d electron .As we move to the right in the group the electrons keep adding to the penultimate 3d shell , and thus Nickel has eight 3d electrons.More the number of 3d electrons, more is the shielding effect. So, the 4s electrons of Nickel experience less electrostatic pull from the protons in the nucleus, than the 4s electrons of Scandium.
Scandium- Ar 3d1 4s2
Nickel – Ar 3d84s2

Due to these two contrasting effects, the size of the transition metals does not increase significantly across the period .
In our next post we shall further continue discussing about other important parameters and their pattern in the periodic table.Till then ,
Be a perpetual student of life and keep learning…
Good day!
References and Further Reading –
1.https://en.wikipedia.org/wiki/Atomic_radius
2.http://chemistry-desk.blogspot.in/2011/06/trend-in-atomic-radii.html
Image Source –