Let us now proceed further and discuss periodic trends in a new parameter – The atomic weight or atomic mass.
The difference between weight and mass –
Generally the terms weight and mass are used synonymously , although their meanings are different.
Mass(measured in g or kg) is a measure of quantity of a substance , whereas
Weight(measured in newtons) is a measure of how gravitational field acts on the mass.
In physics, weight = mass × acceleration due to gravity.
Thus, W=m × g.
For example, our mass remains the same on earth and moon but we would weigh less on the moon than the earth as gravitational acceleration, ‘g’, is different on earth and moon. The quantity g is different because the earth exerts a greater pull on objects, on its surface, than the moon.This difference is attributed to the size of the earth , which is considerably large, than that of the moon.
gearth = 9.807m/s.
gmoon = 1.622 m/s.
In Chemistry, Atomic mass and Atomic weight have different meanings too.
Atomic mass is a relative quantity as it is measured in comparison to the mass of a standard element. Before 1961,physicists and chemists used two different scales to measure atomic weights.Oxygen was used as a standard, as it occurred abundantly in nature and combined with a lot of substances to form oxides.As a result of these two attributes of oxygen,carrying out chemical analysis with oxygen was easier.However, there was a discrepancy in the way physicists and chemists carried out the analysis!
- Chemists were using the oxide formation phenomenon for atomic weight analysis , thus they were considering naturally occuring oxygen as the standard.This naturally occurring oxygen was a mixture of three isotopes of oxygen , namely O-16,O-17 and O-18.
- Physicists were using mass spectrometry for the atomic weight analysis.Thus, they were using pure oxygen -16 isotope as standard.
Thus, there was a lot of confusion associated with the correct method of calculation of atomic weight.
They knew that ,mass of the electron is negligible and
Mass of a proton ≈ Mass of a neutron ≈1 amu (amu = atomic mass unit).
Thus, it was universally decided to choose carbon-12 as a standard as its atom has equal number of protons and neutrons(6 protons and 6 neutrons).Thus today, Carbon-12 , is the standard used for atomic mass measurements and its atomic mass is considered to be exactly 12 amu.
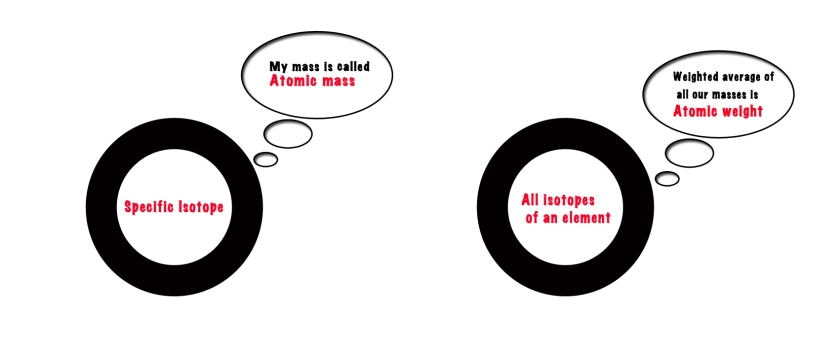
Now, the difference between atomic mass and atomic weight in chemistry is that-
Atomic Mass gives us the mass of a specific isotope of an element. e.g. Atomic mass of C-12 is 12 amu.
Atomic Weight/Relative Atomic mass, Ar ,(the quantity we see on the periodic table) gives us the, weighted average of mass, of all the isotopes of the element. e.g. Atomic weight of Carbon is 12.011 amu .
Note that both atomic mass and atomic weight have same unit – Atomic mass unit , amu.
Definition of Atomic Mass Unit –
It is a unit of mass used to express atomic and molecular weights, equal to one twelfth(1/12th) of the mass of an atom of carbon-12.It is also called Dalton.
∴ 1 amu = 1/12 th of the mass of C-12 ≈ 1.66 × 10 −24grams.
The mass of an atom in amu is roughly equal to the sum of the number of protons and neutrons in the nucleus.
What does the term weighted average mean? How does one calculate atomic weight of an element?
Weighted Average is an average resulting form multiplication of each component by a factor reflecting its importance.
This parameter determines the relative importance of each quantity on the average.The more important or more abundant quantity will affect the average value more i.e the average value will be closer to the relative important quantity.
Let us see how we find weighted average of the atomic masses of the isotopes of the element carbon.
The most abundant isotope of carbon in nature is C-12 isotope followed by Carbon- 13 isotope.98.89% of the carbon found in nature is C-12 isotope and 1.110% is C-13 isotope.The atomic masses of the two are as follows –
|
Isotope |
Abundance |
Atomic Mass |
|
Carbon -12 |
98.89% |
12.0000 amu |
|
Carbon-13 |
1.110% |
13.0034 amu |
Note that the mass of C-13 isotope is more as it contains one neutron (1.0034 amu) extra than the C-12 isotope.Abundance means that out of all the carbon present in nature, 98.89% is C-12 isotope and 1.110% is C-13 isotope.
Now let us find the atomic weight (weighted average) of these two quantities.
98.89% = (98.89)/100 = 0.9889
1.110% = (1.110)/100 = 0.0111
Atomic weight = weighted average = (0.9889 × 12) + (0.111 × 13.0034)
= 12.01113774
= 12.011 (after rounding off).
Thus Atomic weight of Carbon element as on periodic table is 12.011 amu. Note that the weighted average figure,12.011, is close to the mass of the more abundant isotope of carbon , C-12.
References and Further Reading –
1.naomisbell.wordpress.com
2.https://en.m.wikipedia.org/wiki/Relative_atomic_mass
3.https://www.khanacademy.org/science/chemistry/atomic-structure-and-properties/introduction-to-the-atom/v/atomic-weight-and-atomic-mass