The study of chemistry involves understanding how elements react , how chemical reactions occur and the various reasons that drive a chemical change.In this post we shall study a parameter, which is fundamental to understanding the phenomenon of formation of ions from elements – Ionization energy,IE. Why do elements form ions? Why do some elements form positive and some others form negative ions? How do some elements form di , tri or multivalent ions? Let us begin discussing the answers to all these questions..
Generally, elements form ions to attain a stable noble gas configuration.
e.g. – Sodium, Na 1s22s22p63s1. When sodium looses an electron from the 3s orbital , it attains a noble gas configuration i.e Neon configuration.The 3s electron is most loosely bound to the nucleus ,than other electrons (as 3s orbital is farther from the nucleus than 1s ,2s and 2p) and so it is this electron, that comes off easily.

In quantitative terms, the ionization energy of the 3s electron, is the least, amongst all others and thus it is this electron that is preferably lost first.
So, what is Ionization Energy ?
The ionization energy (IE) is defined as the amount of energy required to remove(to infinity) the most loosely bound electron ( valence electron), of an isolated gaseous atom to form a cation.
X + energy → X+ + e−
In simple terms, greater the ionisation energy of the species, harder it is to pull an electron out from it.
Earlier it was also called as ‘Ionization Potential’.The units are eV or kJ/mol. In chemistry, we always talk about ‘Molar ionization energy’ , which corresponds to the energy required to remove the valence electron from one mole of isolated gaseous atoms.
So, henceforth whenever we use the term IE , it would mean ‘molar ionization energy’ i.e the energy per mole necessary to remove electrons from gaseous atoms or atomic ions.
(NOTE – 1 mole of a substance = 6.023 ×1023 entities-atoms/ions of that substance)
Why is gaseous state required for measurement of IE?
The ionization energy for atoms is measured in gaseous state as in this state , the atoms are far apart from each other than the solid or liquid states. This ensures that the atom experiences almost no other force of attraction from neighboring atoms.Thus, measuring ionization energies in gaseous state, is a standard procedure set , for all elements. Ionization energy can also be calculated in liquid and solid states but the value differs in each state.
There is an ionization energy associated with each electron removed from the valence shell. The different ionization energies are as follows –
1)First ionization energy (1st IE ) ⇒ The energy required to remove the first electrons from the valence shell of 1 mole of a species.
2) Second ionization energy ( 2nd IE) – Energy required to remove the second or next loosely bound electron from 1 mole of a species.
3) Third ionization energy (3rd IE) – Energy required to remove the third electron from 1 mole of a species.
It is very obvious that every time , the electron/one of the electrons , with least energy will be preferentially removed. So, the ionization energies after the removal of third electron are too high.
∴IE1 < IE2 < IE3 <IE4 ….. <IEn.
e.g.- Na(g) → Na+ (g) + e–. IE1 = 496 kJ/mol.
Na+(g) → Na2+(g) + e–. IE2 = 4560 kJ/mol.
The ionization energy significantly increases for the second electron !!One can have as many successive ionisation energies as the # of electrons in the atom.
e.g.-
Al(g) → Al+ (g)+ e – IE1 = 577 kJ/mol.
Al+(g) → Al2+(g) + e– IE2 = 1820 kJ/mol
Al2+(g) → Al3+(g) + e– IE3 = 2740 kJ/mol
Al3+(g) →Al4+(g) + e – IE4 = 11600kJ/mol.
So, in order to form Al3+(g)from Al(g), we have to provide 577+1820+2740 = 5137 kJ/mol energy to it ! That is a lot of energy!! Then, why does aluminium form a trivalent ion? It can only do so , if it gets the energy from somewhere. e.g.- In its reaction with fluorine/oxygen it recovers that energy in various changes involving F or O and so it can attain that valency as an ion. Forming a tetravalent ion requires so much more energy and so we don’t see Al3+(g) been formed.
Factors affecting IE –
1)Charge on the species – It is easier to remove an electron from a neutral atom than from a positively charged ion. This is due to the fact that , in a positive ion, the # of protons are more than the # of electrons. Thus, each electron experiences more pull from the nucleus than that in a neutral atom.
2)Distance from the nucleus – As the distance of the electron from increases from the nucleus, the electron pull it experiences from the protons inside the nucleus also decrease.Thus, less energy is required to remove such electrons from their shells.Therefore, with increase in distance from nucleus, the IE decreases.
3)Screening/shielding effect – The electrostatic pull from the nucleus is lessened by the inner electrons( as seen in our earlier post) as the inner electrons act as a shield to the valence electrons , in an atom. Thus,IE decreases.
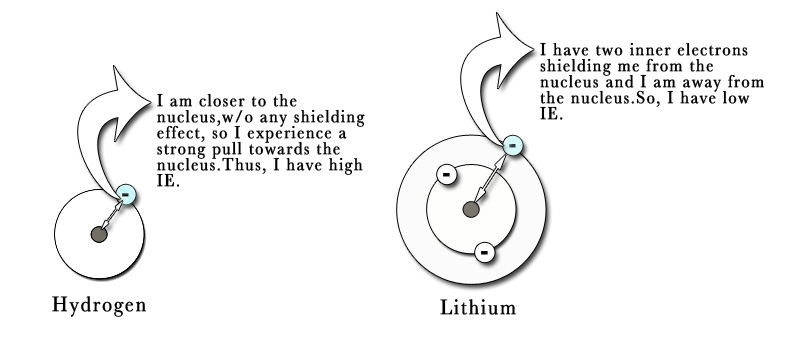
e.g.- First IE for hydrogen = 1310 kJ/mol ← 1s1 is closer to the nucleus , so more attraction. ∴High IE.
First IE for Lithium = 519 kJ/mol ←1s2 2s1 slightly away from the nucleus , so less attraction. ∴Low IE.
IE Trend –
Generally, the ionization energy increases across a period and decreases down a group.
Q: Which of the following element has the highest first ionization energy?
1)As 2)Ge 3)Ga 4) Rb 5) Sr .
A: Ga,Ge,As ⇒ 4th period elements.
Rb,Sr ⇒ 5th period elements.
∴IE of Ga,Ge,As > IE of Rb,Sr.
Now , out of Ga(Group 13),Ge(Group 14) and As(Group 15) , Arsenic will have the maximum IE ,as we know that IE increases across a period.
If we check the values of first IE (from the available database) we find the same result – 
Exceptions /Anomalies-
I)Group 2&3 anomaly-
Group 2 ⇒ Be – B &
Group 3 ⇒ Mg – Al anomaly.
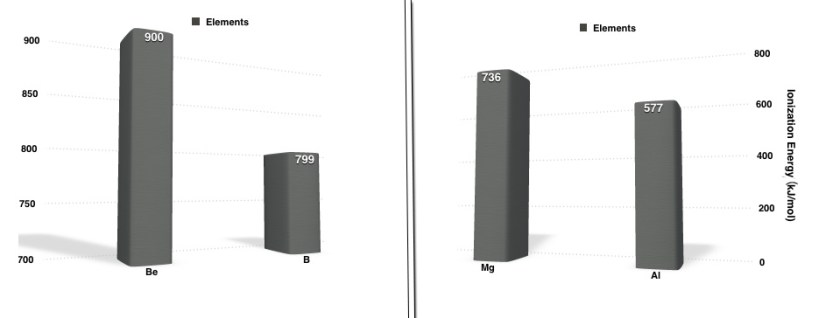
In both the above cases, the s-block elements(Be and Mg) , which come before the p-block elements(B and Al) respectively, have higher IE .This trend defies the general trend – IE increases across a period. The explanation to this deviation can be explained on basis of the electronic configuration of the two sets of elements.
Be(4) 1s2 2s2 ⇒ 2s e– in the s-orbital → closer to nucleus than p. More IE= 900kJ/mol.
B(5) 1s2 2s2 2p1⇒ 2p e– in the p- orbital → farther than s. Thus, less IE = 799 kJ/mol.
Similarly,
Mg(12) 1s2 2s2 2p6 3s2⇒ 3s e– in the s- orbital. Thus, more IE = 736 kJ/mol.
Al(13) 1s2 2s2 2p6 3s2 3p1⇒ 3p e– in the p- orbital. Thus, less IE = 577kJ/mol.
(Note – kJ ⇒ kilos ‘k’ is small letter alphabet .However, Joule is a unit named after the scientist James Joule.So, ‘J’ is a large letter alphabet).
II) Group 5 & 6 anomaly –

As seen in the figure , the 2p and 3p orbitals in oxygen and sulphur have paired electrons, respectively. There is a repulsion between these two electrons as they occupy the same orbital. Thus, one of the these paired electrons is removed first. It is due to the repulsion between the paired electrons , that the IE for Oxygen and sulphur is less than that of Nitrogen and phosphorous respectively.
III) Transition Series anomaly –
As the number of protons increase within a period for each transition series, the first ionization energies of the transition-metal elements don’t change significantly.
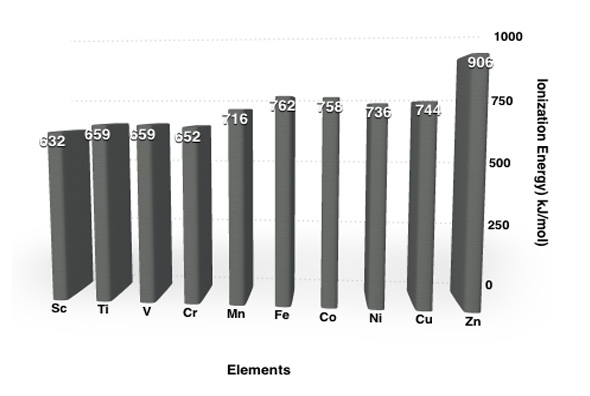
The above figure shows the ionization energies of the first transition series (4th period). Apart from Zinc, all energies have values close to each other.Similar pattern is seen for the second and third transition series too. This can be explained by the following two factors –
|
What happens |
Result |
|
|
Factor 1 |
As one goes from one atom to the next, # of protons increases . So, more attraction. |
IE increases. |
|
Factor 2 |
The electrons however enter the 3d shell only.So, the screening effect for 4s electrons increases. |
IE decreases. |
These two contrasting effects nullify each other.
In case of Zinc, the screening effect is same as copper, but as the nuclear charge is more , the IE increases.
Zn [Ar] 3d10 4s2 IE1 = 908 kJ/mol
Cu [Ar]3d10 4s1 IE1 = 745 kJ/mol
Ionisation energy is measured using spectroscopy.
Ionisation energy values tells us whether a species will act as an electron donor or electron acceptor. Low IE value means , that the electron can be easily lost and thus the element will act as an electron donor.
e.g. – Sodium
High IE value means , that the electron cannot be easily lost and thus the element will act as an electron acceptor.
e.g.– Chlorine
Only when the IE values of two species are very far apart , they tend to form an ionic bond, as in this situation one can donate and other can accept electrons. Elements which do not satisfy this condition, form Polar covalent or covalent bonds.
We shall continue our study on other periodic trends in the successive posts.Till then,
Be a perpetual student of life and keep learning ..
Good Day !
References and Further reading –
1.https://en.wikipedia.org/wiki/Ionization_energy
2.https://chem.libretexts.org/Core/Inorganic_Chemistry/Organometallic_Chemistry/Structural_Fundamentals/Periodic_Trends_of_the_Transition_Metals