We are going to introduce ourselves to a new concept in this post- Electronegativity(χ).Understanding the concept of electronegativity is quite pertinent to the study of chemical bonds and reactions. Let us discuss this new property, and its periodic trends.
Electronegativity(χ) –
Electronegativity(EN) is a property, which describes an atom’s tendency to attract shared pair of electrons towards itself.
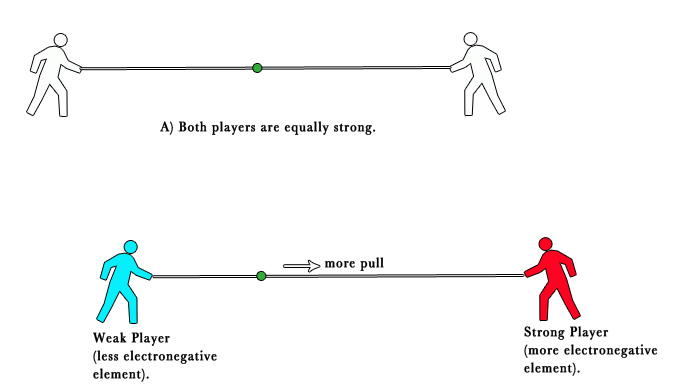
This tendency can be explained with a tug-of-war analogy. In the tug-of-war, if both players have equal strength, the pull from both sides is equal. However, when one player is stronger, he manages to pull the string more toward himself.
Similarly, in a bond, an electronegative element manages to pull the electron density shared between two atoms, more toward itself.
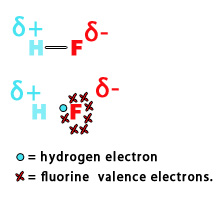
e.g. – Electronegativity of hydrogen = 2.2
Electronegativity of fluorine = 3.98
Thus, in an H-F bond, the fluorine atom pulls the electron density towards itself, introducing polarity in the bond. The adjacent figure shows, how the electron cloud is more toward the fluorine atom than hydrogen. Thus, there is a partial negative charge (𝛅-) on F-atom and a partial positive charge (𝛅+) on H-atom.
Electronegativity is more of a concept and so it does not have units.
Calculation of Electronegativity.
Many attempts were made to quantitatively calculate electronegativity but the most accurate method, which is widely used today, was developed by Linus Pauling in 1932.

Linus Pauling is regarded as one of the two great scientists of all time! He is the only person to be awarded two unshared Nobel Prizes!! He was an American chemist and biochemist who worked on varied subjects like developing electronegativity scales for elements, studying DNA structures, developing dietary supplements, etc
The link to his paper on electronegativity –
http://chemteam.info/Chem-History/Pauling-1932/Pauling-electroneg-1932.html
In his book General Chemistry, he writes –
“It has been found possible to assign to the elements numbers representing their power of attraction for the electrons in a covalent bond, by means of which the amount of partial ionic character may be estimated.”
Fluorine is the most electronegative element with χ= 4.0 and cesium the least with χ=0.7.
When the electronegativity difference is between zero and 0.4, the bond between atoms is covalent. When the electronegativity difference is 1.8 or more, the bond is ionic. When the electronegativity difference is between 0.5 and 1.7, the bond is polar covalent.
| Difference in electronegativity | Type of bond |
| between 0 – 0.4 | covalent bond |
| 1.8 or more | ionic |
| between 0.5 -1.7 | polar covalent |
e.g.-
1)H-H bond is covalent as the EN value difference is zero (both H- atoms have the same EN value).
2)H-O-H bond is polar covalent as χH= 2.2 , χO= 3.44 ; χO – χH = 3.44-2.2 = 1.24. The electronegativity difference between two atoms lies between 0.5 and 1.7. So, the water molecule has a partial positive charge on the H-atoms and a partial negative charge on the O atom.
3) NaCl molecule is ionic as, χNa= 0.93 , χcl= 3.16 , χcl – χNa = 3.16-0.93 =2.23. Here, sodium ion has all the positive charge, and chloride ion is negatively charged.
This explains why the concept of electronegativity is so important – it helps us predict what kind of bond will exist between atoms of a particular set of elements!
Periodic trends in Electronegativity
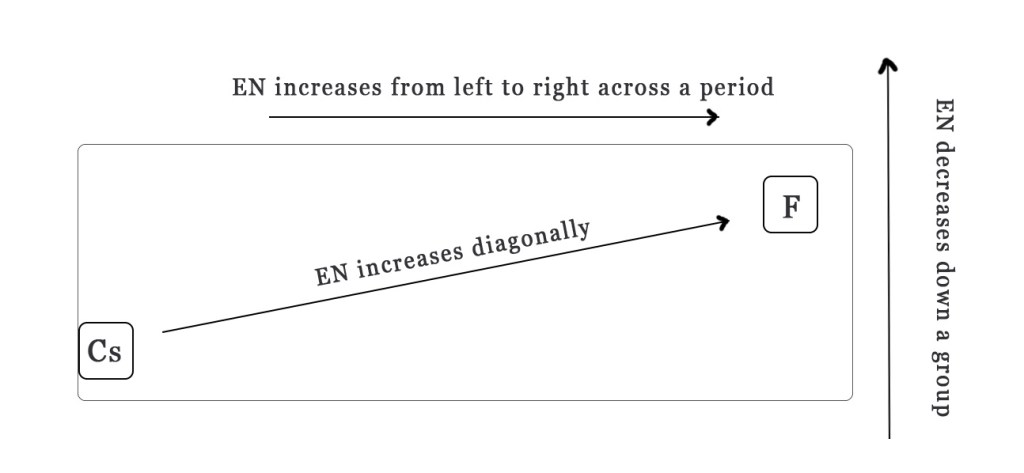
(Note–While remembering the periodic trend in EN, keep the above figure in mind. Fluorine has the highest EN value, so all arrows increase towards F and cesium has the lowest EN value, so all arrows decrease towards it).
Generally, electronegativity decreases down a period and increases across a row. However, the electronegativity trend also shows a diagonal increase as shown in the figure above.
Why do we see this trend?
Down a group–
1)Distance
The valence electrons of an atom are responsible for bonding. As we move down a group, the distance between the bonding pair of electrons (valence electrons) and the nucleus of the atom increases. This is because the size of the atom increases. Thus, the valence electrons are farther from the nucleus. Obviously, as the distance increases, the nuclear pull these valence electrons experience decreases. Therefore, the electronegative values decrease.

As seen in the figure above, the valence electron(red dot) in a sodium atom is farther away from the nucleus than the valence electron of a lithium atom (r1< r2). Thus, the valence electron of sodium is less tightly bound to its nucleus. When a sodium atom shares this electron with another atom, it won’t try to pull the electron cloud towards itself much. Thus, the electronegativity of a sodium atom is low.
2)Screening effect-
Also, the screening effect is greatly increased. So, the pull is greatly reduced.
Across a period –
Across a period, the nuclear charge(# of protons) increases, and the size of the atom decreases. So, the nucleus exerts a greater pull on the bonding electron pairs. Thus, EN values increase.
The diagonal increase of EN values is a combination of the above two factors.
e.g.– Consider Be and B.
EN of Be = 1.5 & EN of B = 2.0 ⇒ (EN value increase across a period)
↓ ⇒ (EN decreases down a group)
EN of Al = 1.5
Thus, beryllium and aluminum, which are diagonally placed in the periodic table, have the same EN values.
The electronegativity of some of the important elements cannot be determined by these trends (they lie in the wrong diagonal). Thus, we need to memorize the following order –
F > O > Cl > N > Br > I > S > C > H > metals
Why are Electronegativity values important?
1)The EN values help us predict whether the bond between two atoms will be ionic, covalent, or polar covalent bond. This helps in understanding their reactions and reaction mechanisms.
2) We can find out bond dissociation energies for a bond with minimum error using EN values. Linus Pauling devised an equation to correctly correlate the two entities.
So how does one use the Pauling scale? How to associate bond dissociation energy with electronegativity?
Pauling observed that the bond dissociation energies of heteronuclear atoms are much more than homo-nuclear atoms.
e.g.– Ed is the bond dissociation energy.
Ed(F2) ≈159 kJ/mol ⇒ 159 kJ/mol energy is required to dissociate/break the F-F bond.
Ed(H2) ≈436kJ/mol ⇒ 436 kJ/mol energy is required to dissociate/break the H-H bond.
Ed(HF) ≈570 kJ/mol ⇒570 kJ/mol energy is required to dissociate/break the H-F bond.
Pauling observed, that the mean of bond dissociation energies(Ed ) of homonuclear molecules i.e H-H and F-F is much smaller than the experimental value of theEd of the heteronuclear molecule H-F.
Mean of Ed=[ (Ed(H2) )+ (Ed(F2) )]/2 = (436+159)/2 ≈ 298 kJ/mol. This value is lower than the actual experimental value of bond dissociation energy of F-F = 570 kJ/mol.
So, he related the difference between the mean value and the experimental value as a measure of the bond polarity in the molecule, which is due to the electronegativity difference between the two atoms forming the molecule( H & F in this case). Thus, he formulated an equation relating bond dissociation energies with EN values as follows –
The difference in electronegativity between atoms A and B is given by:
![{\displaystyle |\chi _{\rm {A}}-\chi _{\rm {B}}|=({\rm {eV}})^{-1/2}{\sqrt {E_{\rm {d}}({\rm {AB}})-[E_{\rm {d}}({\rm {AA}})+E_{\rm {d}}({\rm {BB}})]/2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/716676c0e083fbfdb7460e5fcb90dc857d22d5d9)
where,
χA ⇒ Electronegativity of A
χB ⇒ Electronegativity of B
Ed(AB) ⇒ Dissociation energy of bond A-B in eV.
Ed(AA) ⇒ Dissociation energy of bond A-A in eV.
Ed(BB) ⇒ Dissociation energy of bond B-B in eV.
(eV)-1/2⇒ This factor ensures that the result is dimensionless.
e.g.- For an H-F molecule, the bond dissociation energy can be calculated, using the Pauling equation, as follows –
We know, Ed(H2) ⇒ 436kJ/mol and Ed(F2) ⇒ 159 kJ/mol.
χH= 2.2 and χF= 4.0 (Values obtained from the Pauling scale).
After plugging these values in the equation above, we get Ed(HF) ⇒ 558kJ/mol.
The experimentally observed value of Ed(HF) ⇒ 565kJ/mol.
So, we can theoretically calculate the dissociation energy value with only a 1% error, using the Pauling equation!

3)Electronegativity values can be used to approximately predict the degree of ionic/covalent character of a bond in a heteronuclear molecule.
We shall discuss more periodic trends in the next post. Till then,
Be a perpetual student of life and keep learning …
Good Day!
References and further reading –
1.https://www.chem.wisc.edu/deptfiles/genchem/netorial/rottosen/tutorial/modules/intermolecular_forces/01review/review4.htm
2.https://en.wikipedia.org/wiki/Electronegativity
3.http://www.meta-synthesis.com/webbook/36_eneg/electroneg.html
4.https://chem.libretexts.org/Core/Physical_and_Theoretical_Chemistry/Physical_Properties_of_Matter/Atomic_and_Molecular_Properties/Electronegativity
Image source –
1. By Library of Congress – http://www.notablebiographies.com/Ni-Pe/Pauling-Linus.html, Public Domain, https://commons.wikimedia.org/w/index.php?curid=17802529
2.https://chemistry.stackexchange.com/questions/33611/why-are-there-peaks-in-electronegativities-in-d-block-elements
3.http://www.meta-synthesis.com/webbook/36_eneg/electroneg.html
Thank you for your blog 🙂
LikeLike
🙂 🙂
LikeLike