In the last two posts, we discussed the valence bond theory (VBT) at length. The VBT theory is not foolproof and we shall discuss its drawbacks shortly. However, before proceeding any further, let us first get to know the VSPER model, which helps us understand the shape of the molecules.
Valence shell electron pair repulsion model (VSEPR)
VSEPR model (pronounced “vesper“) is a way proposed to explain the shape of the molecules. According to this model, the valence electron pairs surrounding the atoms will orient themselves in such a way, as to minimize electron-electron repulsion between them.
Thus, it is the valence electron pairs that determine the geometry of the molecule.VSEPR is not a theory but just a model, giving us a way of predicting the shape of molecules.
It is important to know the difference between bonding pair of electrons and non- bonding /lone pair of electrons, before learning this model. Let us discuss these concepts.
THE LONE AND BONDING PAIR OF ELECTRONS.
Lone pair of electrons ⇒ electrons that are present on a single atom, which is not shared with any other atom.
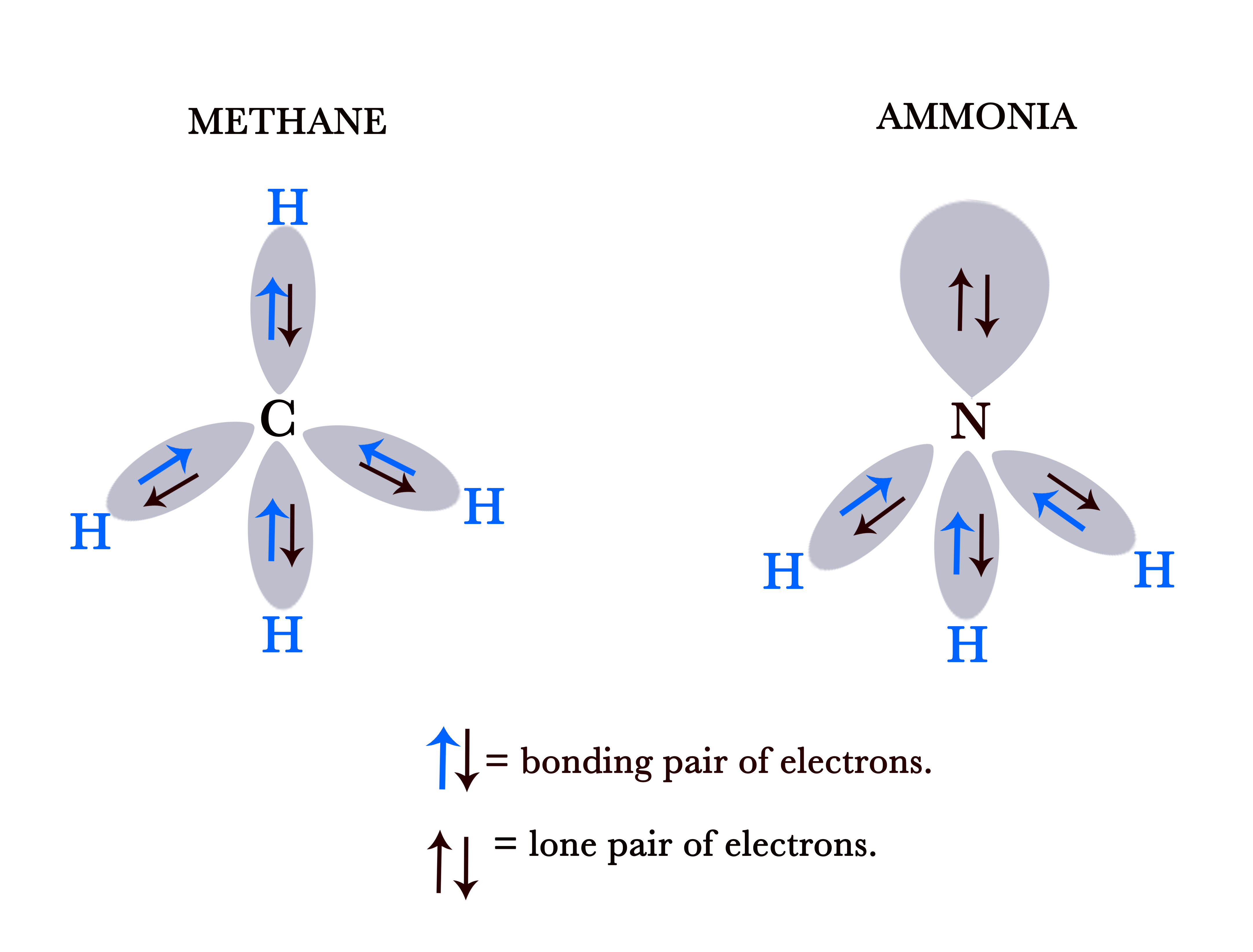
Bonding pair of electrons ⇒ electrons that are shared between the two atoms, forming the bond.
e.g.- As seen in the figure above, all electrons are bonding pairs of electrons in the methane (CH4) molecule. These electrons are shared between the two atoms, namely – Carbon (C) and Hydrogen (H).
However, in the ammonia (NH3) molecule, there are two electrons that are not shared. They reside only on the nitrogen atom. These are the lone pair of electrons. (We shall discuss why these lone pairs of electrons are not shared, in a later post, when we study the hybridization of orbitals).
ELECTRON DOMAIN
The electron domain refers to the region the electron pairs occupy in space. In the VSEPR model, we talk about the electron domains and their effect on the geometry of molecules.
The AXE method.
VSEPR model uses the AXE method to count the # of electron pairs on the central atom, in a molecule.
A ⇒ central atom.
X ⇒ bonding atom.
E ⇒ # of lone pairs.
STERIC NUMBER (SN) = no.of atoms bonded to the central atom + no. of lone pair of electrons.
∴SN = X+ E
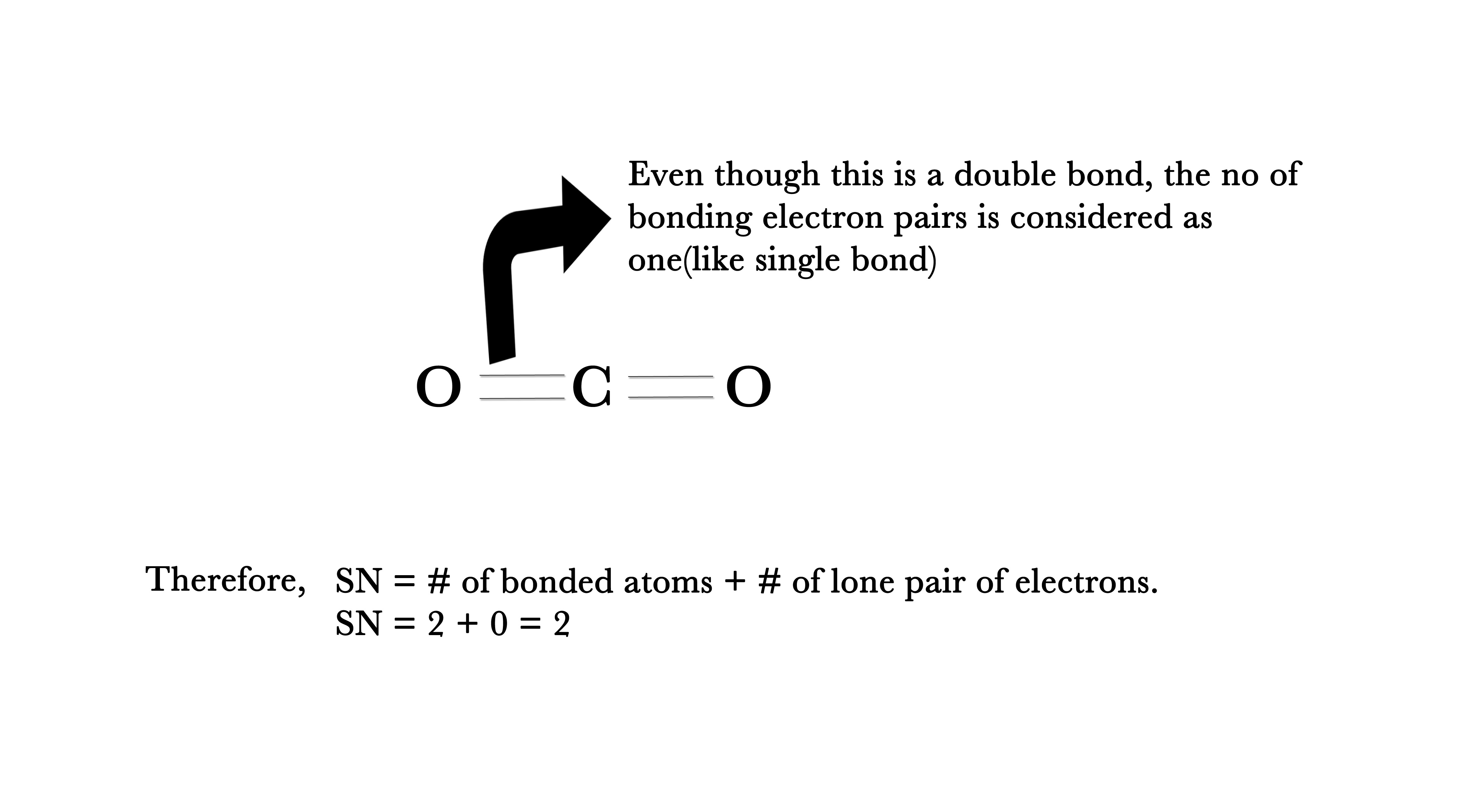
NOTE –In VSEPR, while calculating SN, double and triple bonds are also treated as single bonds, which means that for a double/triple bond, the no.of bonding pair of electrons is just one.
e.g. – In, CO2 molecule , SN = 2 + 0 = 2.
Determining the geometry using the VSEPR model.
We begin by drawing Lewis structures for molecules( Refer to post no 53 to know more about Lewis structures).
We draw all the electron pairs and lone pairs around the central atom (this is usually the least electronegative atom). Each lone pair and each electron pair are one single group. Each bond counts as a single group even if it is a double or triple bond.
e.g. –
Central atom: Carbon
Valence electrons on the central atom: 4
2 Os contribute 6 electrons each: 12
Total: 16.
Divide by 2 to give electron pairs 8 (4 lone pairs which are NOT on the central atom, so they won’t be considered, and two double bonds are considered as a single bond).
8 – 4 – 2 = 2
| VSEPR geometry(AX2E0) | Linear |
According to this model, electron pairs occupy positions in space such that there is minimal electrostatic repulsion between them. The geometries are given in the following table –
|
Electron groups (# Bond pair) |
Geometry |
Bond angle |
Figure |
Examples |
Structure |
|
2 (AX2E0)
|
Linear |
1800 | 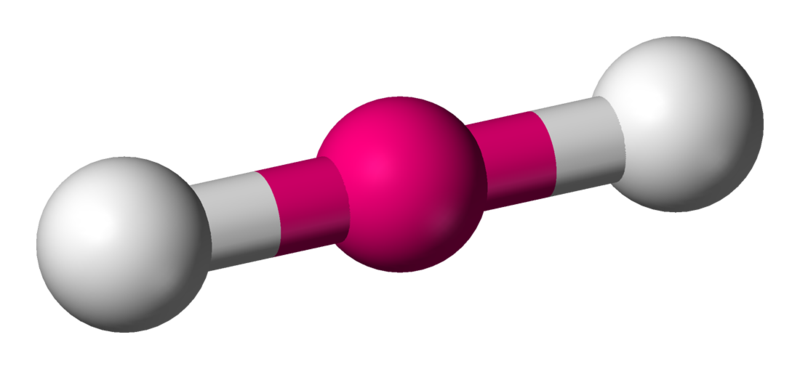 |
CO2,BeCl2,BeH2 |  |
|
3 (AX3E0)
|
Triagonal planar |
1200 |  |
BCl3 |  |
|
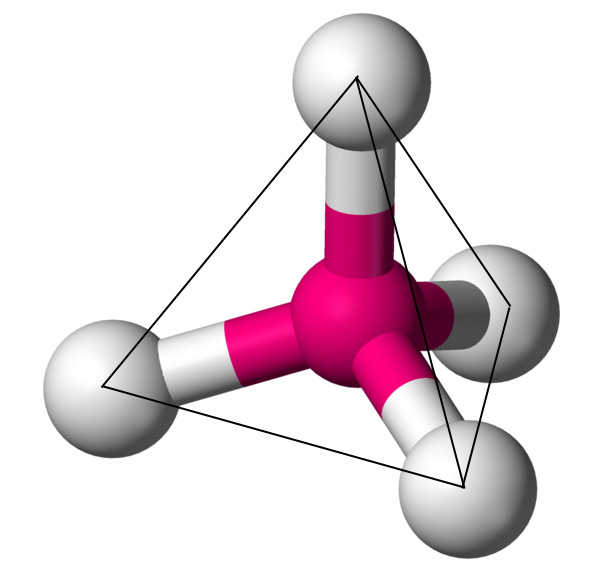
4 (AX4E0) |
Tetrahedral |
109.50 |  |
CH4 | 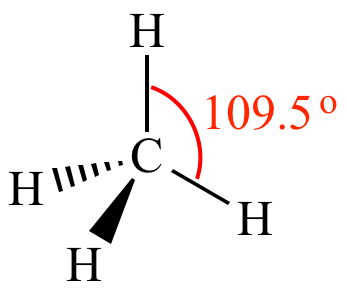 |
|
5 (AX5E0) |
Trigonal bipyramidal |
900,1200 | 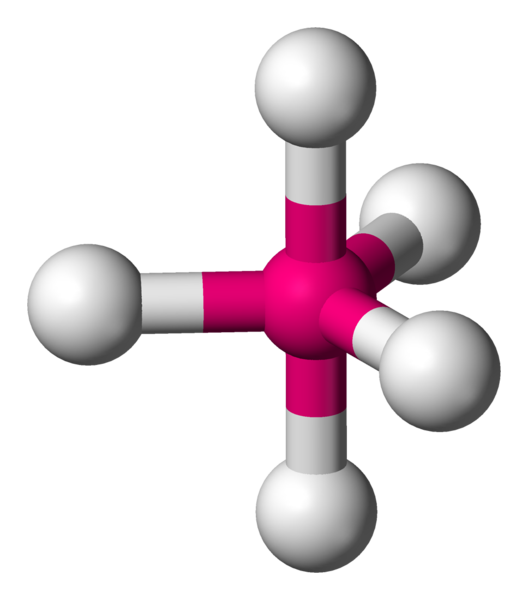 |
PCl5 |  |
|
6 (AX6E0) |
Octahedral |
900 |  |
SF6 | 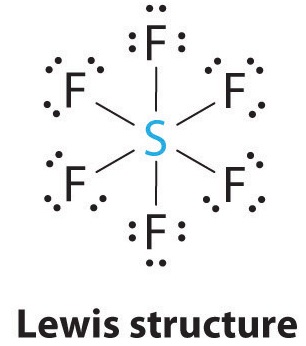 |
All above examples have no lone pair of electrons and so it’s E0.
Why do these shapes get their respective names?
- Linear means in a line – when molecules have all atoms in a straight line, the geometry is termed linear.

- Trigonal planar – when three atoms occupy three vertices of a triangle(trigonal) and they all lie in one plane, the geometry is trigonal planar.

3. Tetrahedral – When atoms occupy the four corners of a tetrahedron, the geometry is tetrahedral.

- Trigonal bipyramidal – In this geometry, the atoms form two pyramidal structures(on top and bottom to the plane containing three atoms) and the three atoms (all in one plane) lie at the vertices of a triangle.

5. Octahedral – In this geometry, atoms occupy six vertices of an octahedron.


These were the geometries of molecules with no lone pair electrons. In our next post, we shall study what happens with the introduction of one or more lone pairs of electrons in a molecule. Till then,
Be a perpetual student of life and keep learning…
Good day!
Image source –
2)https://chem.libretexts.org/Textbook_Maps/General_Chemistry_Textbook_Maps/Map%3A_Chemistry%3A_The_Central_Science_(Brown_et_al.)/09._Molecular_Geometry_and_Bonding_Theories/9.2%3A_The_VSEPR_Model
3)https://www.google.co.in/search?biw=1280&bih=648&tbm=isch&sa=1&ei=Tmp4WuzlNYnxvgSUjp24Cg&q=tetrahedron+&oq=tetrahedron+&gs_l=psy-ab.3..0l10.154534484.154535008.0.154535800.3.2.0.1.1.0.240.430.0j1j1.2.0….0…1c.1.64.psy-ab..0.3.447…0i67k1.0.5wb5twmvRm4#imgrc=A0mqO1cd5ncZGM:
4)https://www.google.co.in/search?biw=1280&bih=648&tbm=isch&sa=1&ei=J1l9WoObL8X6vATww6bABg&q=octahedron+pink+color&oq=octahedron+pink+color&gs_l=psy-ab.3…0.0.1.5467.0.0.0.0.0.0.0.0..0.0….0…1c..64.psy-ab..0.0.0….0.0wlGGSTHU_0#imgdii=qlSQEyXBQhlcnM:&imgrc=lcTFD9aP3gNZRM:
5)https://www.google.co.in/search?biw=1280&bih=648&tbm=isch&sa=1&ei=J1l9WoObL8X6vATww6bABg&q=octahedron+pink+color&oq=octahedron+pink+color&gs_l=psy-ab.3…0.0.1.5467.0.0.0.0.0.0.0.0..0.0….0…1c..64.psy-ab..0.0.0….0.0wlGGSTHU_0#imgrc=lcTFD9aP3gNZRM: