CONCEPT OF HYBRIDIZATION
As we studied in the last post, Linus Pauling introduced an imaginary concept of ‘hybridization of atomic orbitals to explain the valency and geometry of certain molecules.
Hybridization essentially means mixing. This term is often used in biology, where animal and plant hybrids are created by cross-breeding two different species, to get a hybrid fruit/vegetable/plant/ animal of better quality.

Similarly, in this theory, atomic orbitals are mixed to form new orbitals. This process of mixing atomic orbitals of slightly different energies in an atom to form a new set of orbitals of equivalent energies with maximum symmetry is called hybridization.
How do orbitals hybridize?
Hybridization involves the following steps –
STEP # 1 – FORMATION OF EXCITED STATE.
In the atom – that undergoes hybridization – electron/s from the lower energy valence orbitals/sub shells get promoted to higher energy vacant valence subshells i.e they get excited to a higher energy state.
Energy is released when extra bonds are formed i.e formation of extra bonds is an energetically favored process. The electrons in the lower energy valence subshell, get excited by absorbing energy. This excitation is favored on account of the fact that more bonds can be formed, thus lowering the energy of the system. The energy absorbed to excite the ground state electrons to a higher energy level is compensated by the energy that is released during bond formation. Note that theoretically, these steps happen one after another. However, in reality, it is a combined process.
e.g.- In a carbon atom, there are only 2 unpaired valence electrons in the 2p subshell.
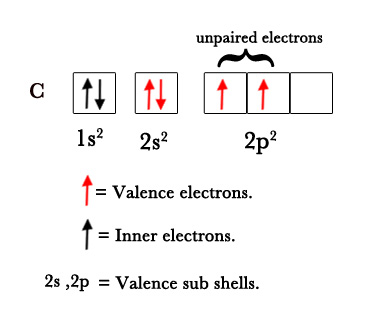
C (6) 1s2 2s2 2p2.
During hybridization, one electron from the 2s subshell gets promoted to the 2p level as seen in the following figure –

Now carbon atom has four unpaired electrons, which can form four bonds. These four valence electrons have different energies. In reality, however, all four bonds have similar energies. This can be explained by the next step in the hybridization process.
STEP # 2 – MIXING AND RECASTING OF ATOMIC ORBITALS.
In this step, the atomic orbitals of the atom mix to form a new equivalent number of hybrid orbitals of equal energy. This mixing of orbitals to form new orbitals is called hybridization. The orbitals having higher energy are slightly demoted in energy and the orbitals with lower energy are slightly promoted to higher energy, thus making all the orbitals of equal energy.
(NOTE – Here ‘orbitals’ means only the ‘valence orbitals’, which actually take part in bond formation)
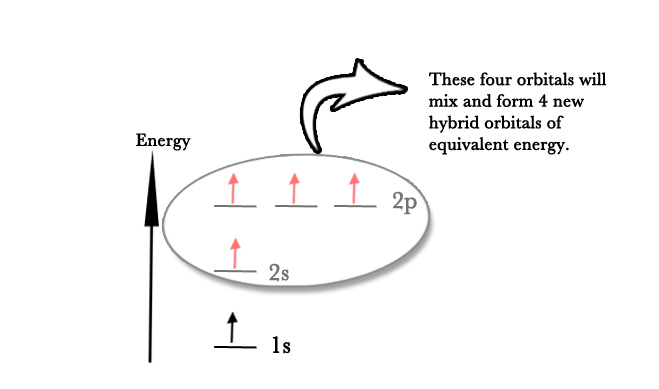
As seen in the above figure, the 2s and 2p orbitals DO NOT have the same energy. So, Linus Pauling suggested that the 2p orbitals be slightly demoted in energy and 2s orbitals be promoted in energy, and all orbitals are mixed to form four hybrid orbitals of the same energy. These new orbitals are however different from 2s and 2p orbitals. The four new hybrid orbitals are four sp3 orbitals. These new hybrid orbitals form 4 equivalent bonds with other species as seen in practice.
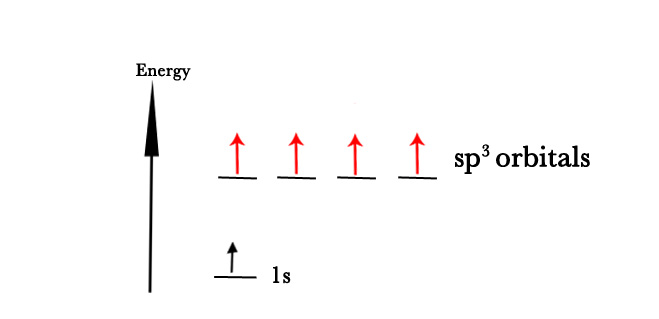
In the above example, we have considered a carbon atom with one 2s and three 2p orbitals. Thus, we get four new sp3 orbitals.
Similarly, sp2 and sp orbitals are also formed in molecules having one s, two p, and one s, one p orbitals respectively.
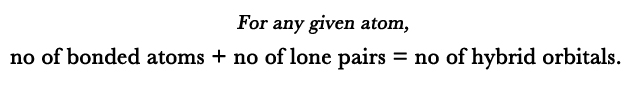
|
No. of hybrid orbitals |
# of unhybridized orbitals involved |
Type of hybridisation |
|
4 |
one s + three p |
sp3 |
|
3 |
one s + two p |
sp2 |
|
2 |
one s + one p |
sp |
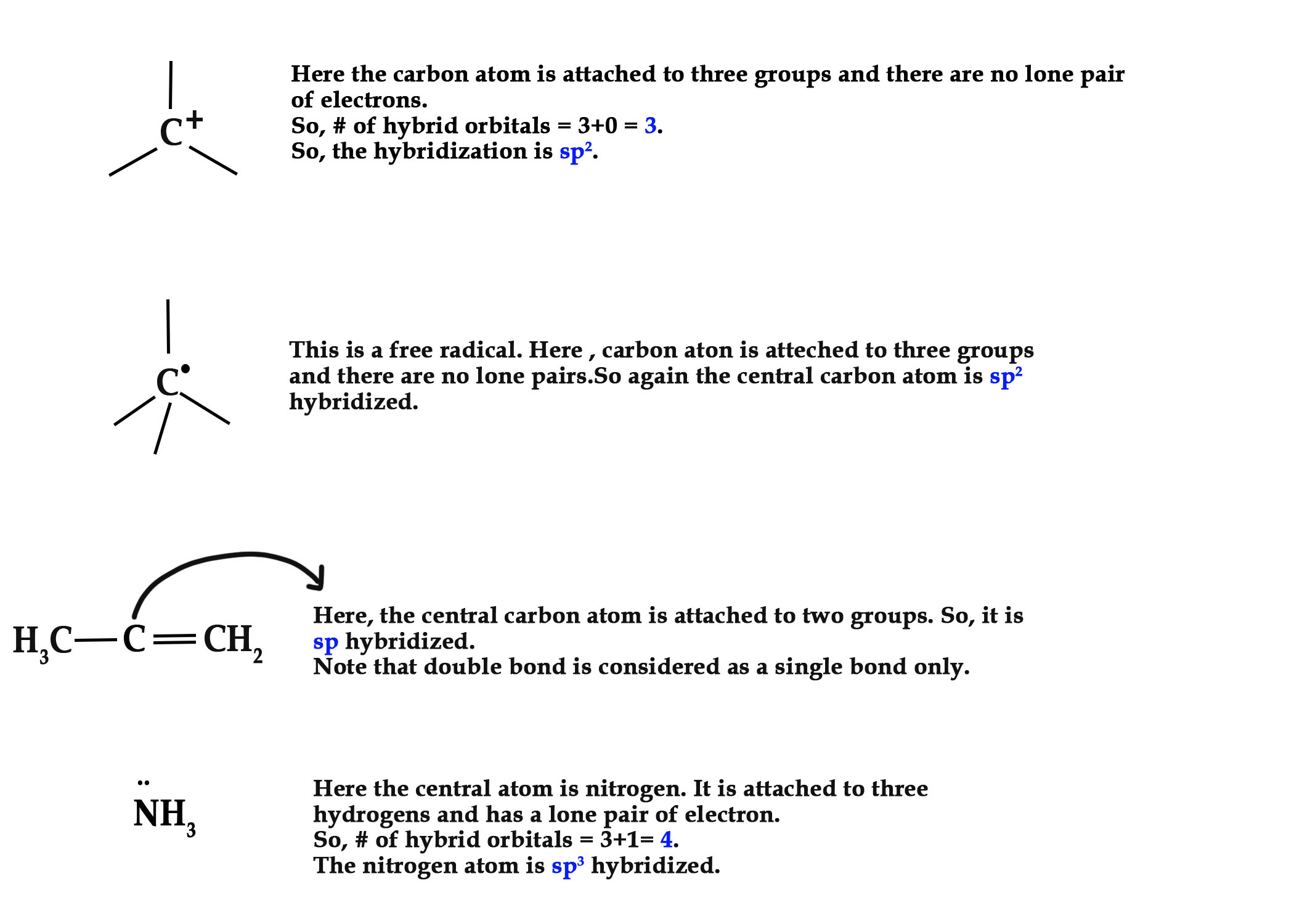
However, there is an exception to this rule.
If an atom –
I) has one or more lone pairs and
II)is attached to a sp2atom, then that atom is also sp2 hybridized.
e.g. –

STEP #3 – RE-ORIENTATION

The new hybrid orbitals have a different shape than the parent orbitals. These new orbitals orient themselves in space in order to minimize repulsion between each other as seen in the VSEPR model. So, the s-orbital is spherical in shape, and p- orbitals are dumbbell shaped. However, the sp3 orbitals are neither spherical nor dumbbell-shaped, they have a shape intermediate between the two (tetrahedral).
Important Features of the hybridization theory –
- Hybridization takes place only between atomic orbitals of nearly the same energy. Thus, 2s and 2p orbitals can hybridize, but 2s and 4p cannot.
- For a given atom,# of hybrid orbitals = (# of bonded atoms) + (# of lone pairs) EXCEPTION – single bonded terminal atoms.
e.g.- Formyl chloride, CHClO.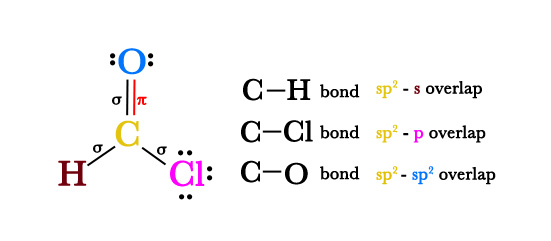
- As seen in the above figure, the carbon atom is sp2 hybridized. How did we know that? Use the formula given above –
# of hybrid orbitals = (# of bonded atoms) + (# of lone pairs)
The carbon atom is bonded to three atoms namely H, Cl, and O and it has no lone pair of electrons.
∴ # of hybrid orbitals = 3 + 0 = 3.
Thus, the carbon atom is sp2 hybridized. H and Cl are single-bonded atoms so they are not hybridized. The 1s and 3p valence orbitals of H and Cl respectively, directly participate in bond formation with carbon’s sp2 orbitals.
The oxygen atom is bonded to a carbon atom and has two lone pairs of electrons. so again using the above formula,
# of hybrid orbitals = (# of bonded atoms) + (# of lone pairs)
# of hybrid orbitals for O atom = 1+ 2 = 3.
Thus, the oxygen atom is sp2 hybridized too. So, one C-O σ bond is sp2 – sp2 hybridized. The other double bond is a π bond. The π bonds, formed by p-p overlap are not formed by hybridization. - It is not necessary that all the atomic orbitals in an atom take part in hybridization. Hybrid orbitals only form sigma bonds. Orbitals involved in π bond formation (double/triple bonds) do not take part in hybridization. Also, single bonded terminal atoms are not involved in hybridization.
- Hybridization takes place only in orbitals, electrons are not involved in it.
- Each hybridized orbital is more concentrated on one side of the nucleus so that greater overlapping can take place and thus a stronger bond is formed. Thus, the hybrid orbitals form stronger bonds.
We can conclude that –
| Type of hybridization | Hybrid Orbitals | # unhybridized p orbitals |
|
sp3 |
Four sp3 orbitals |
none |
|
sp2 |
Three sp2 orbitals |
One p orbital |
|
sp |
Two sp orbitals |
Two p orbitals |
What exactly happens while mixing atomic orbitals?
Consider a simple example of sp hybridization. One s orbital and one p orbital overlap to form an sp hybrid orbital. We know from our ‘quantum mechanics’ posts (post no 32 and 33), that an orbital is a region, where the probability of finding the electron( which behaves as a wave) is the most. So, an orbital is a mathematical function, which describes the behavior of the electron in it.
When two atomic orbitals overlap, there is constructive as well as destructive interference between the electrons (behaving as waves) in those two orbitals. In sp hybridization, the larger lobe of the hybrid orbital shows the region of constructive interference and the smaller lobe (the tail region) shows the area of destructive interference.
Constructive interference → Probability of finding the electron is highest.
Destructive interference → Probability of finding the electron is the lowest.

Bond angle.
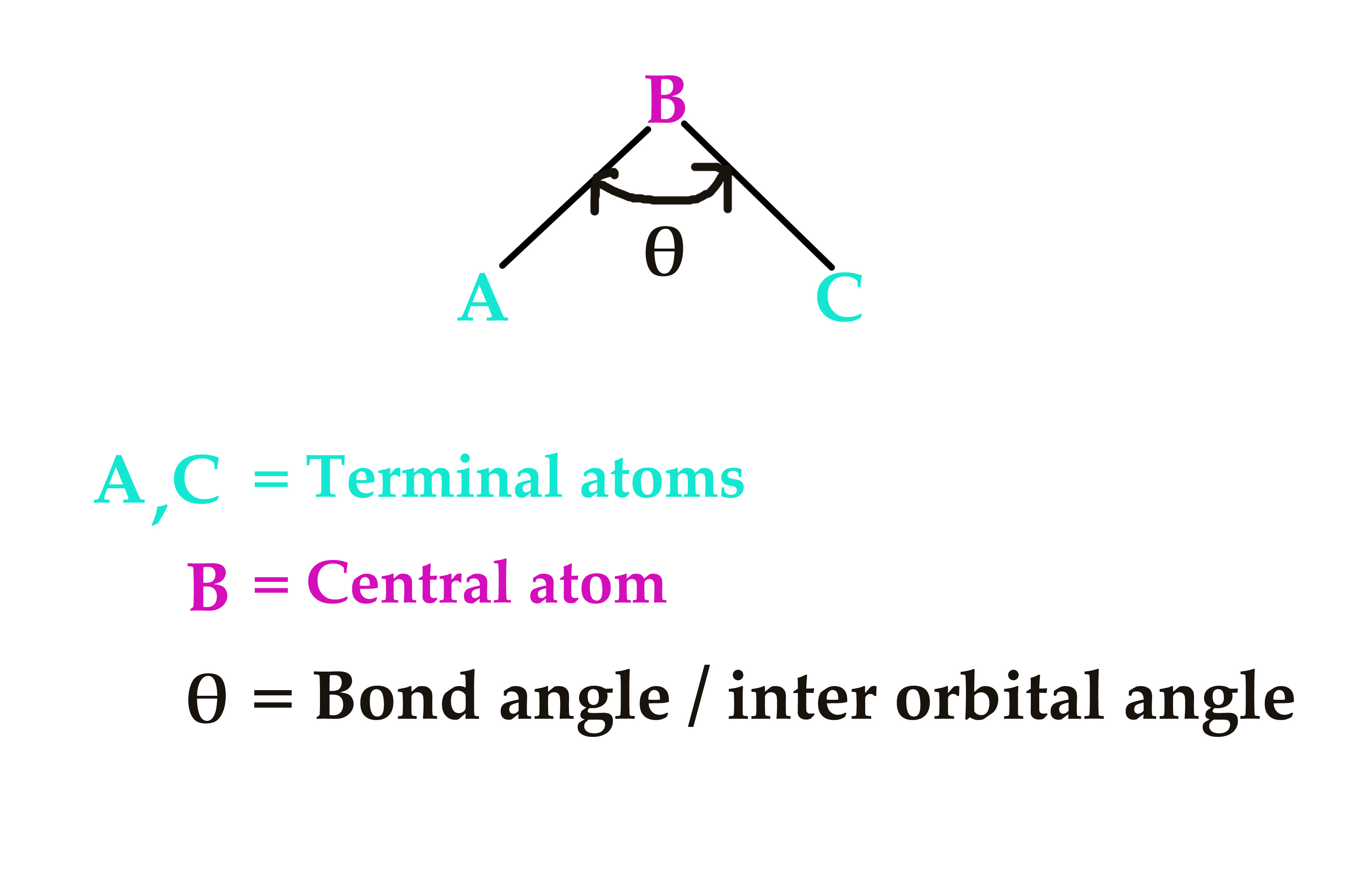
The angle formed between the centra and terminal atoms in a molecule is called the bond angle or inter orbital angle. It is represented as θ,
In our next post, we shall study molecules formed by the hybridization process. Till then,
Be a perpetual student of life and keep learning…
Good Day!
References and Further reading –
1.https://www.emedicalprep.com/study-material/chemistry/chemical-bonding/hybridization/