sp3 hybridization
The process of mixing of one s- orbital with three p- orbitals in an atom to form four sp3 hybrid orbitals of equivalent energy is called sp3 hybridization.
EXAMPLE 1 – METHANE (CH4).
We generally draw the structure of methane as follows –

This structure would imply that methane has bond angles of 90º and 180º and the molecule has planar geometry . However, methane is NOT a planar molecule.
In the methane molecule, the central carbon atom is sp3 hybridized.
Carbon (6) in ground state 1s2 2s2 2p2
C(6) 1s2 2s1 2p3. One 2s electron promoted to 2p orbital.
Then, 2s and 2p hybridize to form four sp3 orbitals.
Later, the methane molecule is formed with four hydrogen atoms. The electron in the hydrogen atom is in the 1s orbital. Thus, this is an sp3 – s overlap.


Methane molecule has tetrahedral geometry(bond angle = 109.5º ) as predicted by the VSEPR model. In the tetrahedral shape, all four hybrid orbitals are as far away as possible in space, from each other. Thus, there is minimum repulsion amongst them, which renders the system stable.

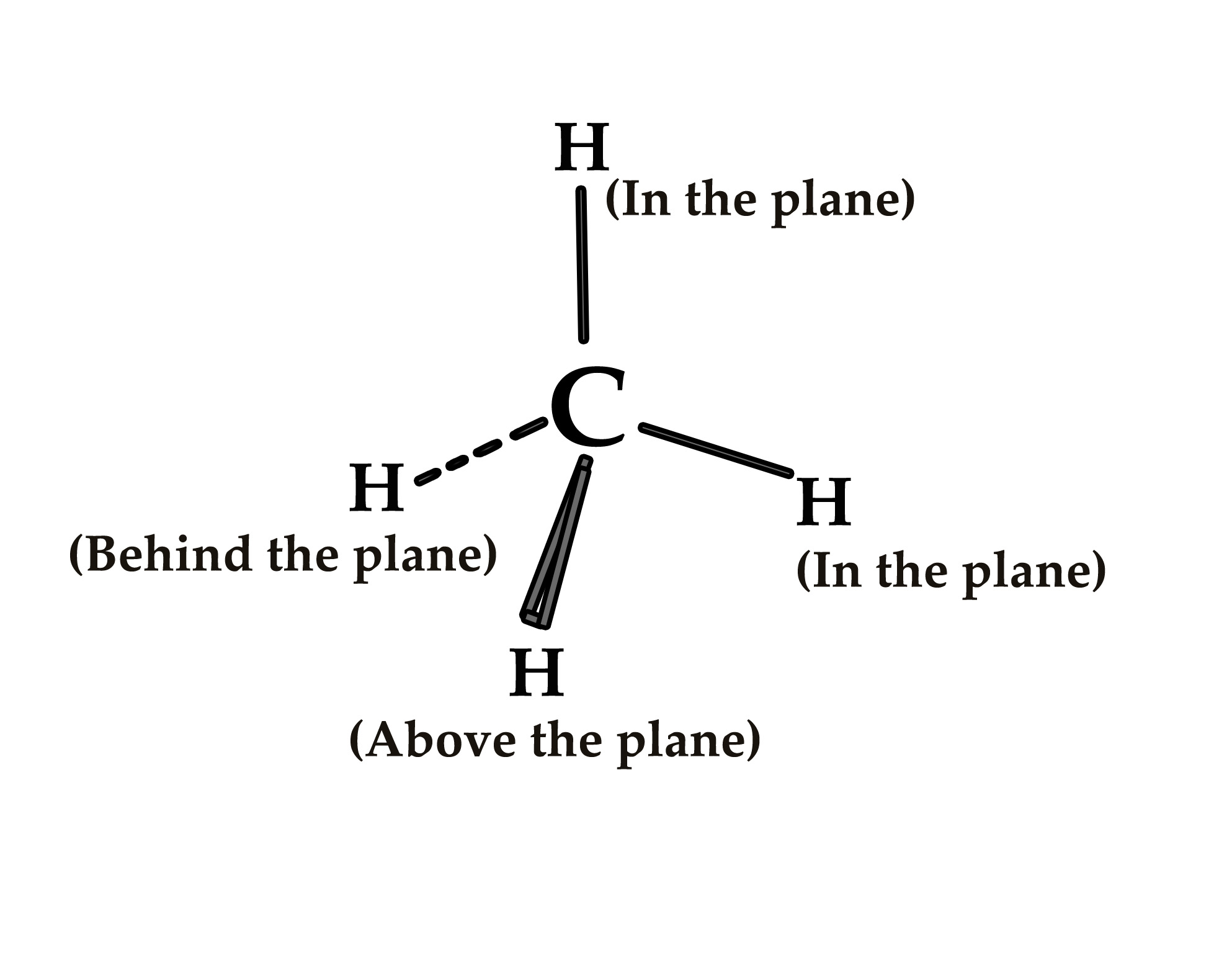
The molecule is represented as –
This is the convention used for representing three-dimensional (3D) molecules-
i) The substituents which are above the plane of the paper, towards the observer are attached to the central atom by a solid wedge line.
ii) Substituents in the plane are shown by just a line.
iii) Substituents that recede behind the plane, are shown by dotted/ dash lines.
This convention helps us imagine the three-dimensional structure, even if the figure is drawn in two dimensions.
EXAMPLE 2 – AMMONIA (NH3).
In the ammonia molecule, the central atom of nitrogen is sp3 hybridized.

Nitrogen (7) in the ground state 1s2 2s2 2p3. Here no electrons are excited to higher energy levels. The 2s and 2p hybridize to form four sp3 orbitals. One 2p orbital has a lone pair of electrons and this orbital DOES NOT take part in bonding . Thus, lone pairs of electrons are also referred to as ‘non-bonding’ electron pairs. Later, the ammonia molecule is formed with three hydrogen atoms. The electron in a hydrogen atom is in the 1s orbital. Thus, this is an sp3 – s overlap.

The one pair needs more space and so it pushes the other orbitals away from it and closer to each other .Thus,the lone pair changes the geometry of the molecule from tetrahedral to trigonal pyramidal. Subsequently, the bond angle changes from the regular 109.5º to 107.3º.

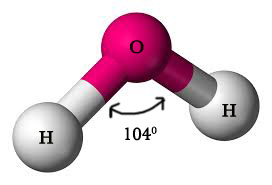
EXAMPLE 3 – WATER (H20).
In water molecule, the central atom oxygen is sp3 hybridized .
Oxygen (8) in ground state1s2 2s2 2p4. Here no electrons are excited to higher energy levels.
There are two unpaired electrons in two 2p orbitals of oxygen atom in ground state and it forms two bonds with two hydrogen atoms.Then , why is hybridization theory needed to explain the formation of water molecule?
If the two bonding electrons (one each in two 2p orbitals ) were in unhybridized orbitals they would have been at 90º to each other. However, the observed bond angle is 104°.Thus, the concept of hybridization is necessary to explain the observed bond angle.
The 2s and 2p hybridise to form four sp3 orbitals. Later, the water molecule is formed with two hydrogen atoms. The two half filled sp3 orbitals overlap axially with two 1s hydrogen orbitals.Thus, this is a sp3 – s overlap.


Water molecule has a V-shaped geometry. The two lone pairs occupy more space than the bonding pairs and thus the distortion from tetrahedral shape. The bond angle is lesser than methane and ammonia molecule too.
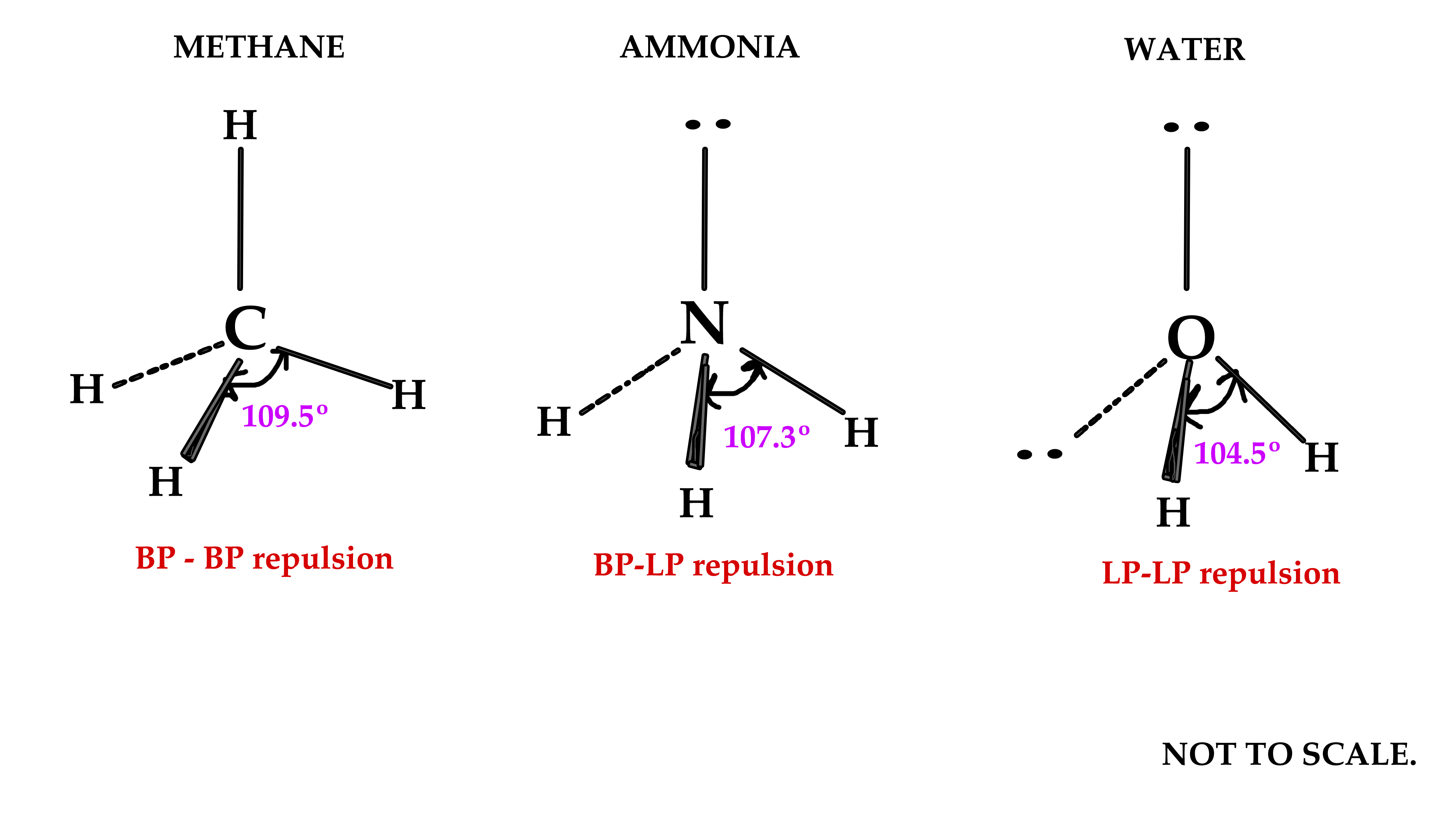
An sp3 hybrid orbital has 25% s- character and 75% p-character .(This concept is discussed in detail in post #68).

In our next post we start talking about the next kind of hybridization. Till then,
Be a perpetual student of life and keep learning…
Good Day !
Image source –
- https://en.wikipedia.org/wiki/Tetrahedral_molecular_geometry
- https://www.google.co.in/search?biw=1200&bih=526&tbm=isch&sa=1&ei=7GQzW5PLFYWOvQTjiKXAAg&q=molecular+geometry+bent&oq=molecular+geometry+&gs_l=img.3.0.0i67k1l3j0l3j0i67k1l4.13681.17350.0.18595.19.10.0.9.9.0.113.976.7j3.10.0….0…1c.1.64.img..0.19.1017…35i39k1.0.FxSCjo9NhZI#imgrc=ZFMyapxWzqsFxM:
- https://www.google.co.in/search?biw=1200&bih=526&tbm=isch&sa=1&ei=_2QzW8j2K5CgvQSz7p3AAQ&q=molecular+geometry+triagonal+pyramidal+&oq=molecular+geometry+triagonal+pyramidal+&gs_l=img.3…3071823.3078794.0.3079022.20.20.0.0.0.0.221.1937.18j1j1.20.0….0…1c.1.64.img..0.3.410…0j0i67k1j0i30k1j0i8i30k1.0.FCDwaBmg3-4#imgrc=vp9rJ4wir5z3BM:
Thank you so much! I understand clearly right now 🙂
LikeLike