sp2 hybridization.
The process of mixing one s- orbital with two p- orbitals in an atom to form three sp2 hybrid orbitals of equivalent energy is called sp2 hybridization.
EXAMPLE – ETHYⅬENE / ETHENE
In the ethene molecule, the central carbon atom is sp2 hybridized.
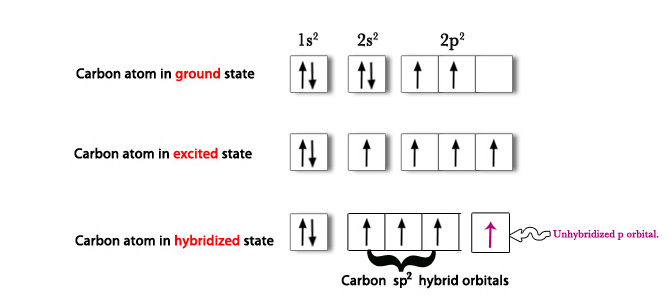
Carbon (6) in ground state 1s2 2s2 2p2
One 2s electron is promoted to 2p orbital→ C(6) 1s2 2s1 2p3
Then, 2s and two 2p hybridize to form three sp2 hybrid orbitals.
Two sp2 hybrid orbitals of each carbon atom overlap to form sp2 – sp2 bond. This is the sigma bond between two carbon atoms.
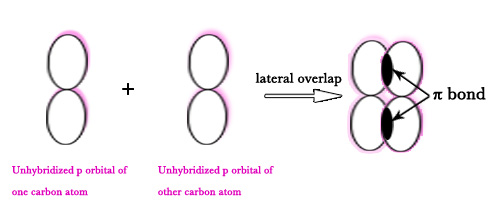
However, in this case, a π bond is formed too. The unhybridized pz orbitals (the p-orbitals which DO NOT take part in hybridization) of both the carbon atoms lie perpendicular to the hybridized orbitals. They overlap laterally/sideways to form a π bond.

The unhybridized p orbital lobes, which overlap laterally to form one π bond, are shown in pink color in the following figure –
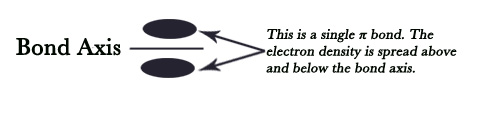
NOTE – i)Diagrammatically, the π bond is always shown above and below the sigma bond. Though it’s shown in two places(these are two regions where p orbital lobes overlap) it symbolizes one π bond only. The two regions show that the electron cloud is above and below the bond axis.
The ethene molecule is formed with 4 hydrogen atoms. As we already know that the electron in a hydrogen atom is in the 1s orbital. Thus, this is an sp2– s overlap.
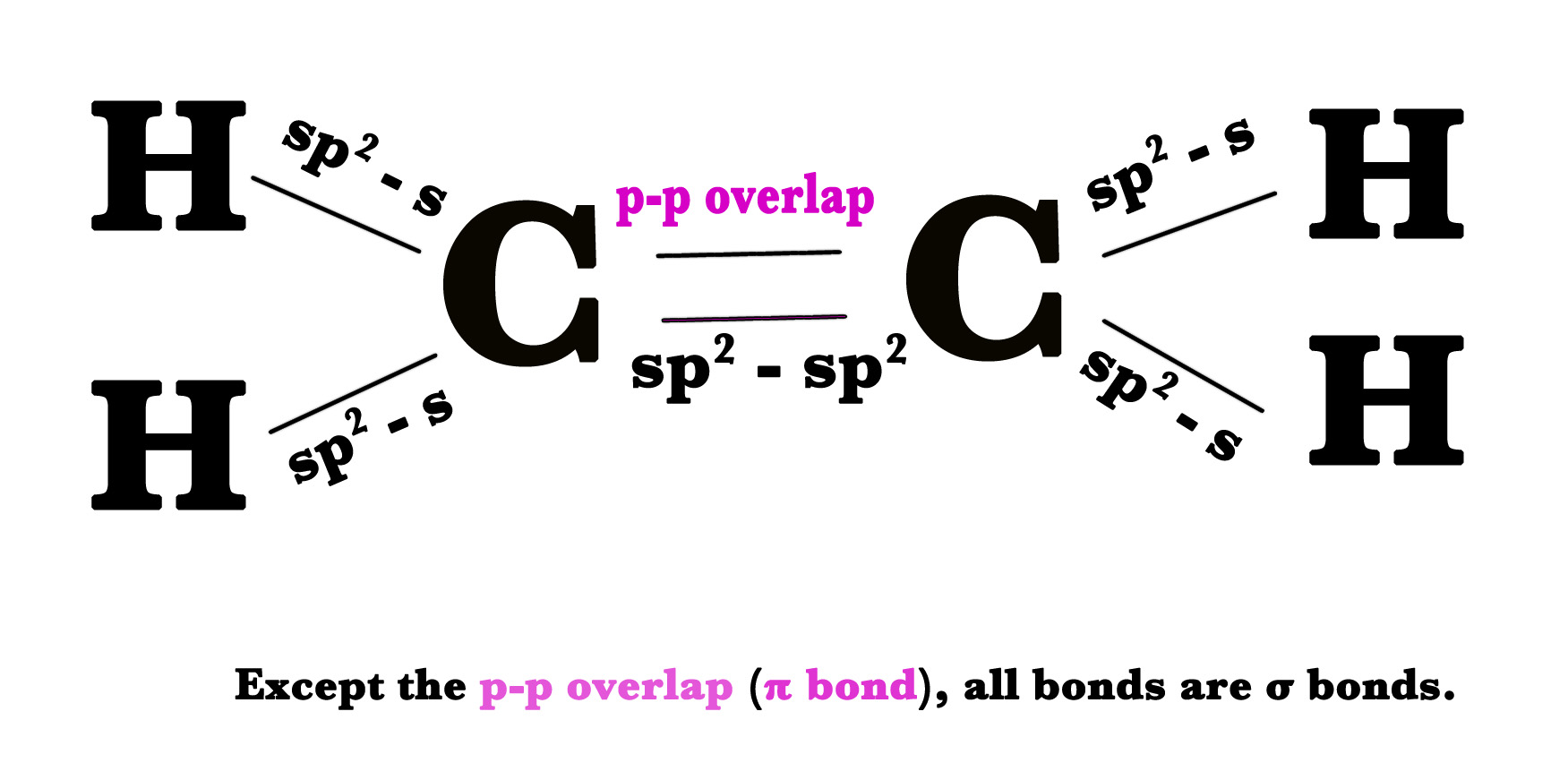
Pictorially the above bonds can be shown as follows –
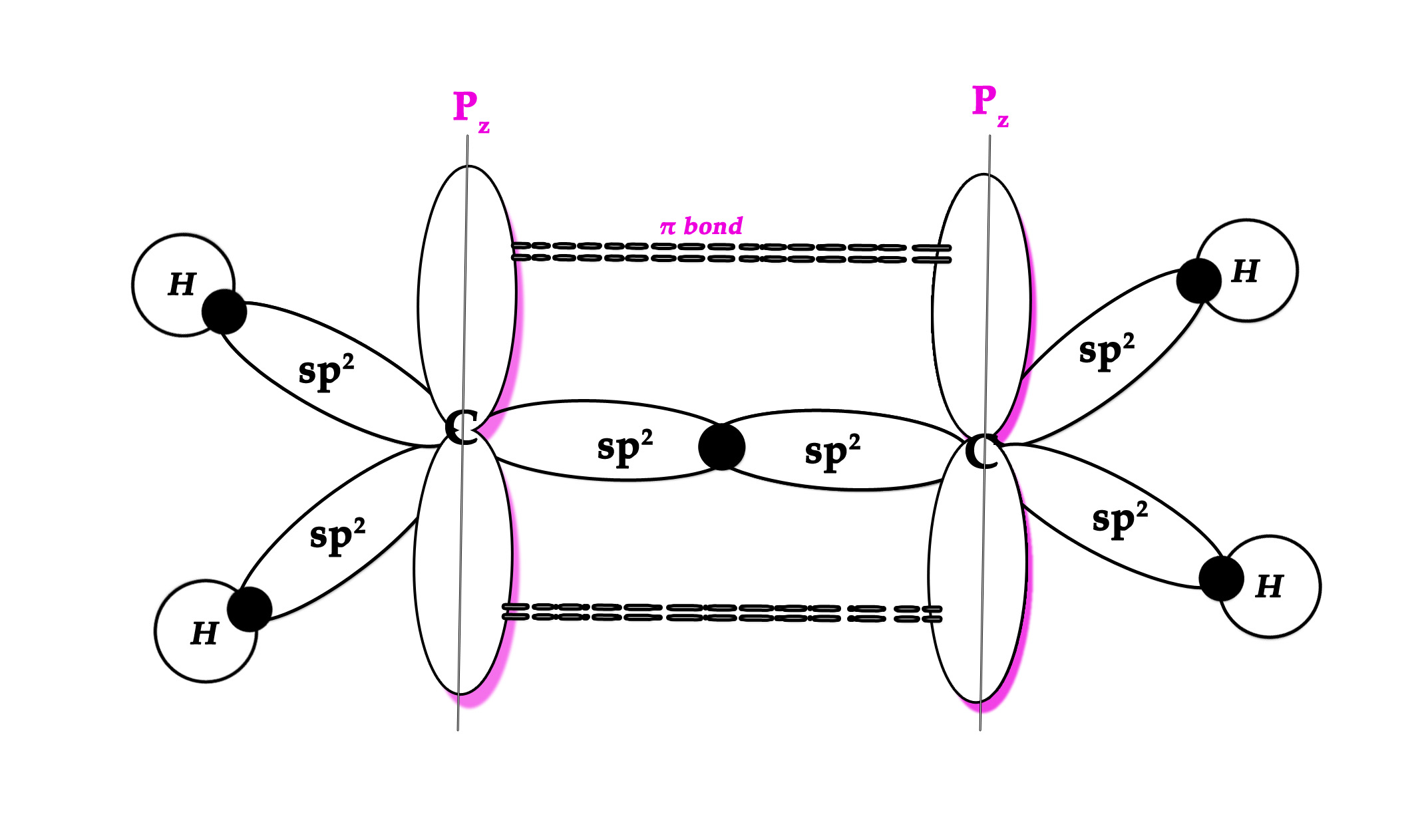
NOTE – The three hybrid orbitals point towards the corners of a triangle at 120o to each other. They all are in the same plane – in the plane of the bond axis. The pz orbitals are perpendicular to the bond axis.
sp2 hybridization occurs when a carbon atom is attached to 3 groups. In the above example, each carbon atom is attached to two hydrogen atoms and another carbon atom.
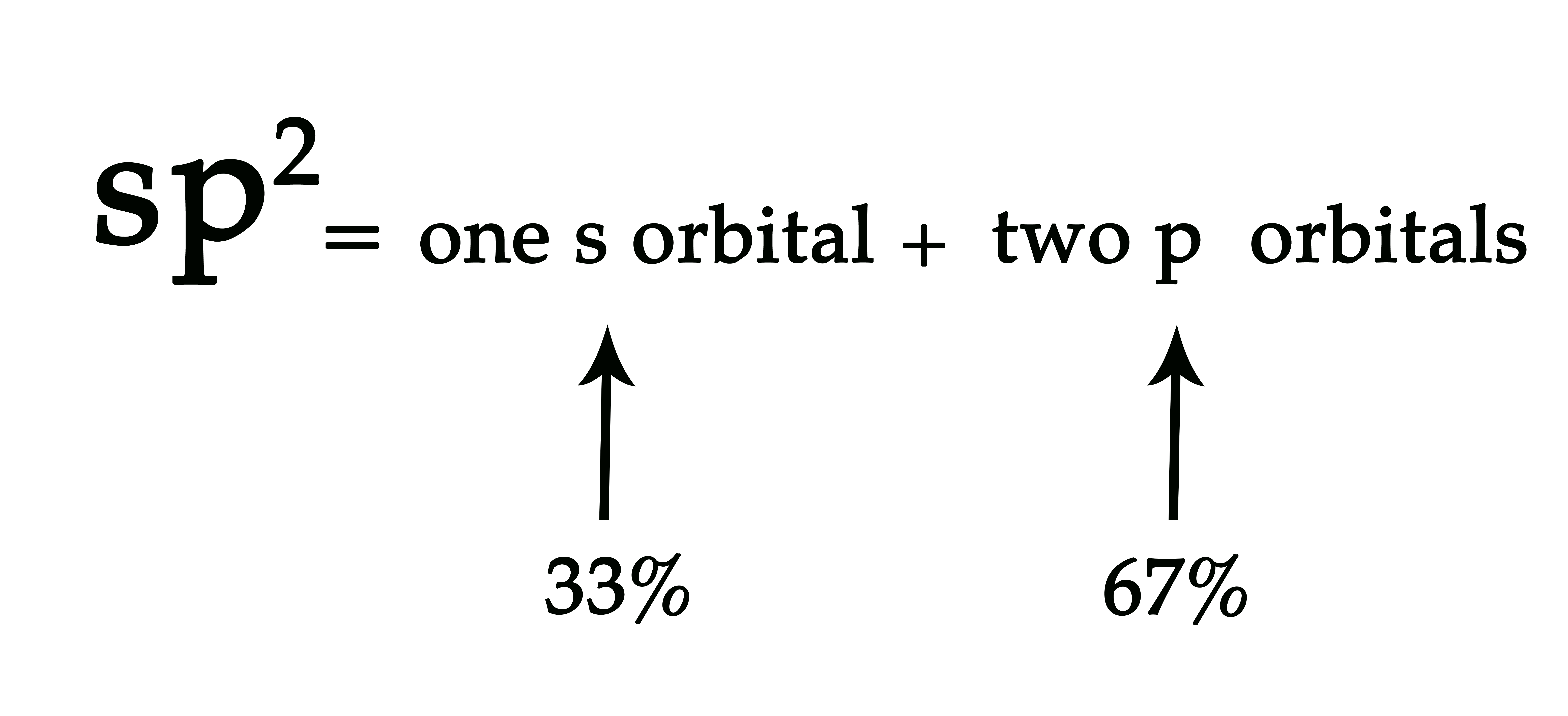
An sp2 hybrid orbital has 33% s character and 67% p character.
In our next post, we shall discuss sp hybridization. Till then,
Be a perpetual student of life and keep learning…
Good day!
References and further reading –