We have already studied that the angle between the central and terminal atoms of a molecule is called a bond angle. In this post, we study how various factors affect the measure of this angle.
Factors affecting the bond angle (θ).
The factors affecting the bond angle are –
i)Hybridization or % s character – As the s-character in the hybrid orbital increases, the bond angle increases too.

As the s-orbital is the closest to the nucleus, the electrons in it are tightly bound. These electrons, which are closer to the central atom repel each other the most. This repulsion results in shifting the bonds as far away as possible, thus increasing the bond angle.
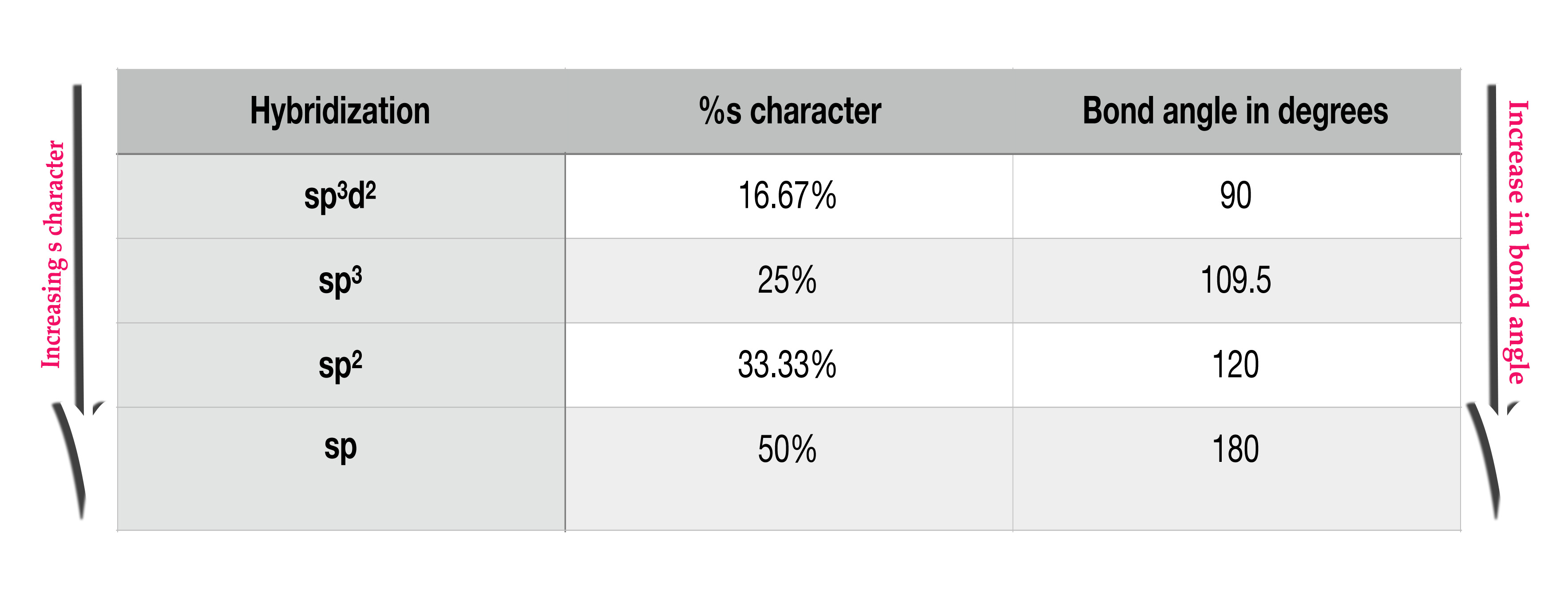
As seen in the above table, as the %s character in a hybrid orbital increases, the bond angle increases too ( both the side arrows, besides the above table, point in the same direction → ∴directly proportional )
ii) Lone pair of electrons – We have already studied how lone pairs change the bond angles in post 58.
Intensity of relpusion → LP – LP > LP- BP > BP – BP.
So, a molecule with more lone pairs will have smaller bond angles, owing to the fact that, the lone pairs occupy more space and thus they push the other orbitals closer together than expected.
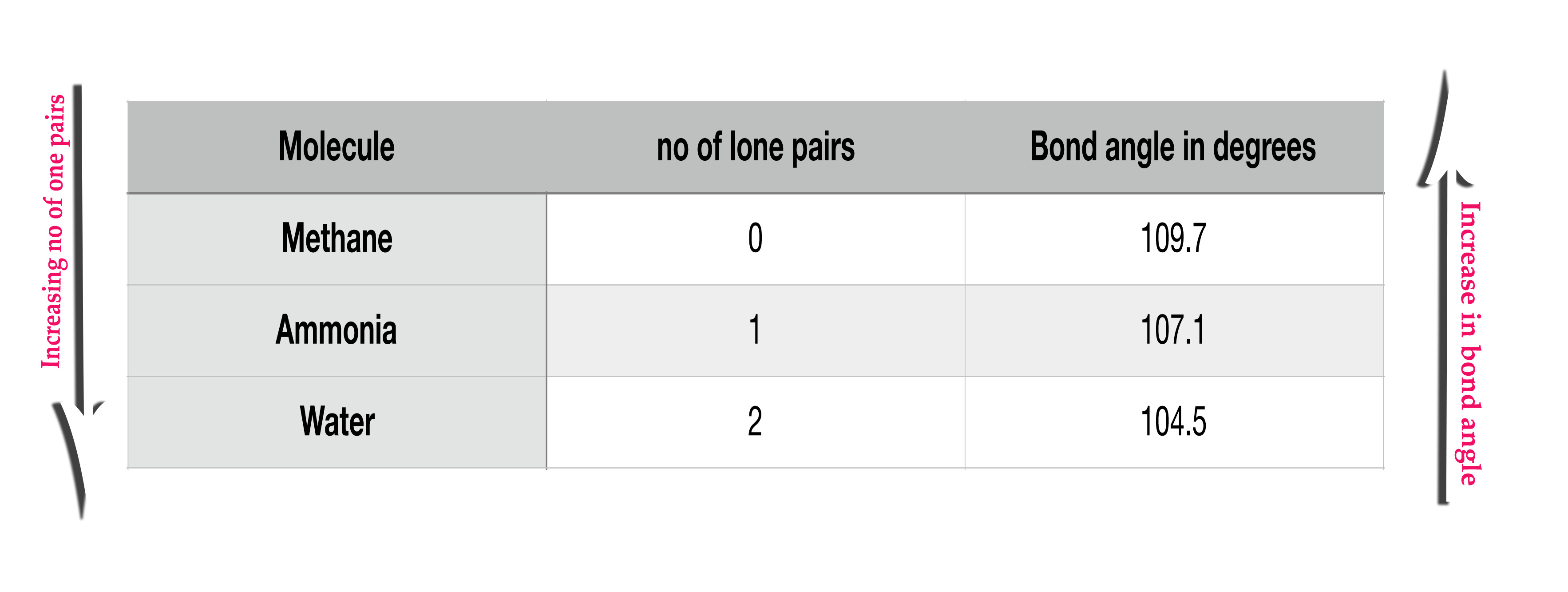
(Both side arrows, besides the above table, point in opposite directions.)
iii) Electronegativity(χ) of the central atom –
Electronegativity is directly proportional to the bond angle i.e if electronegativity of the central atom decreases, the bond angle decreases.
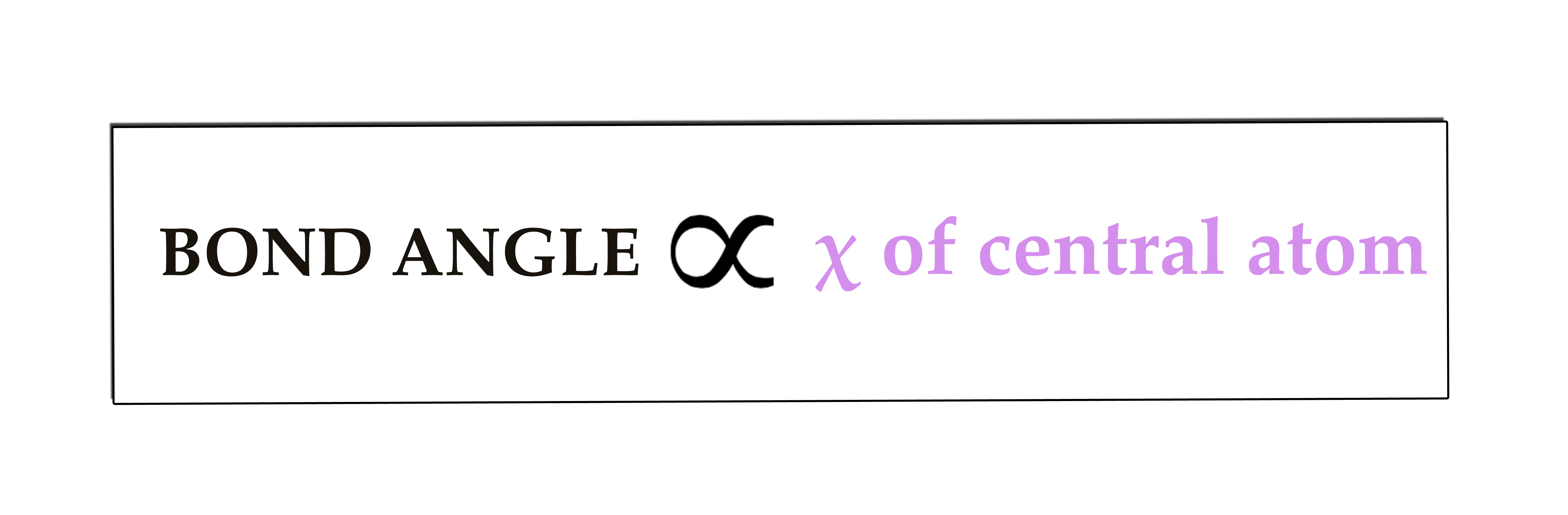
This phenomenon is seen because in an electronegative element,
A] the electrons are closer to the nucleus of the central atom.
B] the size of the most electronegative element is less compared to the other elements.
So, the charge density on the central atom is high. Thus, there is more repulsion between the electrons, which try to be as far away as possible from each other. This results in increasing the bond angle.
Consider the following molecules – All four molecules have the same hybridization and the same no of lone pair of electrons. Only the central atom is different in each of these molecules.
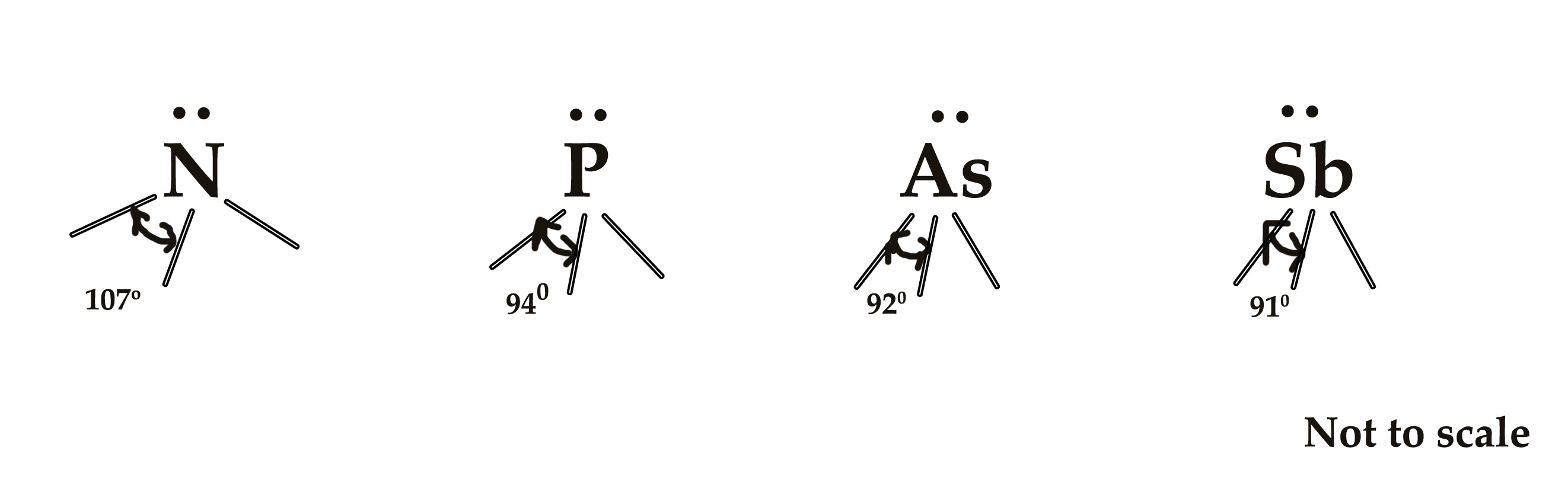
The central atoms in all the four molecules are group 15 elements. If we look at the periodic table, we know that the order of electronegativity is as follows –
N> P> As >Sb
So, Nitrogen is the most electronegative among the other three. This can also be related to what we have learned in post 46 – as we move down a group, electronegativity decreases as the size of the atom increases.
As we see in the diagrams above, the bond angle decreases as the electronegativity decreases and vice versa. Thus, the electronegativity of the central atom and the bond angle is directly proportional to each other – when one increases the other increases too and vice versa.
iv) Electronegativity(χ) of the substituents –
The bond angle is inversely proportional to the electronegativity of the substituents. A more electronegative substituent pulls the electrons towards itself thus decreasing the charge density on the central atom. This results in a decrease in bond angle.

We have already studied this in post no 58 –
Bonding pairs of electronegative substituents occupy less space than the electropositive ones as these electrons are strongly attracted by the electronegative atom(so they are closer to its nucleus) . As the bonding pair of electrons is attracted towards the more electronegative substituent, the charge density on the central atom decreases. Thus, the repulsion between two BP’s becomes less. Thus, the adjacent hybrid orbitals can be near each other and so the bond angle becomes less. So, basically, as the repulsion ( think of it as hatred for each other ) is less, the hybrid orbitals can be closer to each other. In presence of a lone pair, the LP – BP repulsion further pushes the orbitals with BP closer, which decreases the bond angle further.
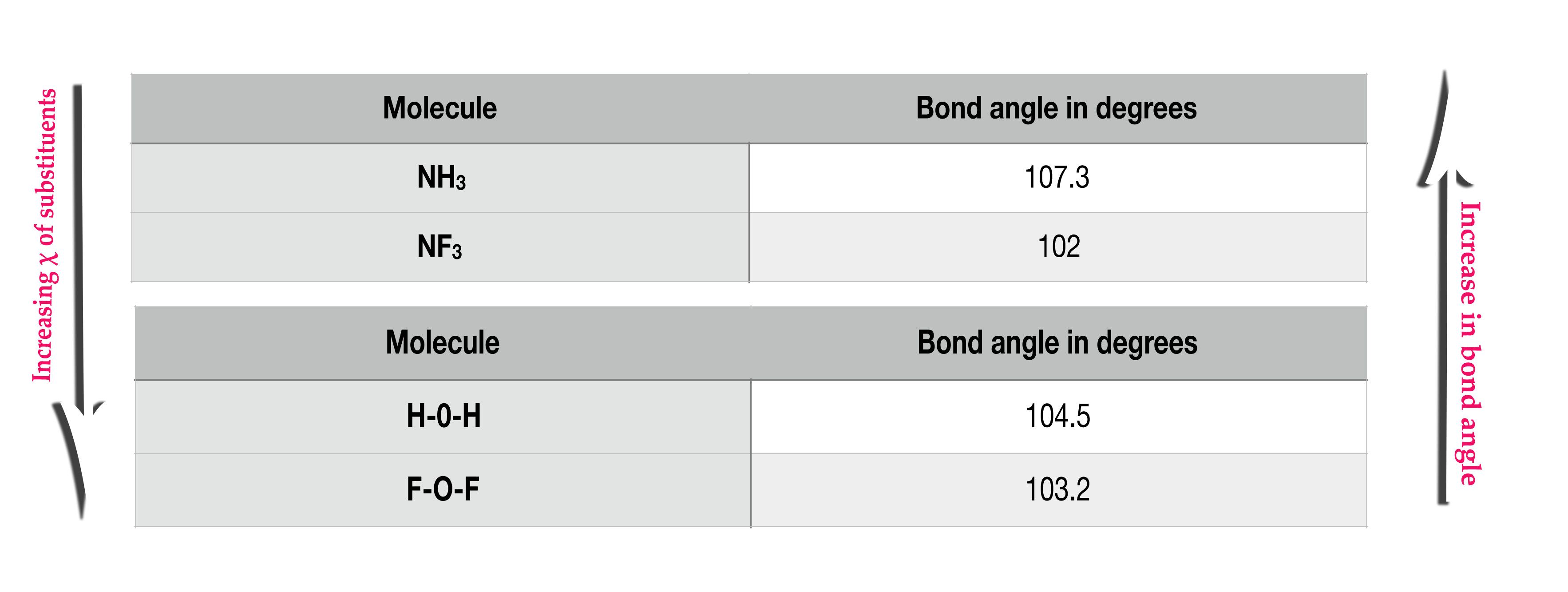
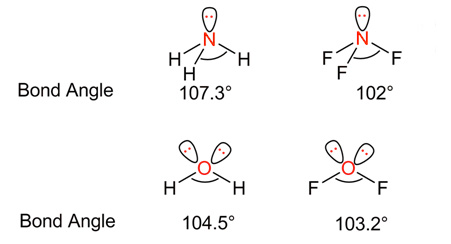
In the above example, fluorine is more electronegative than hydrogen. So, compounds containing F atom(NF3 and OF2) have less bond angle than those with H atoms(NH3 and H20).
In our next post we start discussing a new rule , which was quite recently stated. This rule shall help us in predicting the geometry of various molecules.
Be a perpetual student of life and keep learning…
Good day !
Such a nice post!!
LikeLike
Thank you 🙂
LikeLike
Thanks 🙂
LikeLike
thankyou so much
LikeLike
My pleasure !!!
LikeLike
thank you very much, you are an angel
LikeLike
Thanks !
LikeLike
Thank you Man
LikeLike
🙂
LikeLike
Very clear
LikeLike
🙂 🙂 🙂
LikeLike
Thank u maam, this helped me a lot !!!
LikeLike
I am glad I could help you in some way 🙂
LikeLike
Thanks
LikeLike
Welcome 🙂
LikeLike
Thank you 😊
LikeLike
Thank you so much 🙂
LikeLike
thx a lot it was very clear
LikeLike