In this post, we shall begin by constructing MO diagrams for various molecules. We begin with the simplest of all homonuclear diatomic molecules – The H2 molecule. However, before proceeding, we will study the rules of drawing a MO diagram.
Rules of building a MO diagram
Rules of building a MO diagram are –
- The atomic orbitals are shown on the far left and far right of the diagram. The MOs are drawn between these two ends.
- The Y-axis shows energy of the orbitals.
- Electrons get filled in MOs in accordance with Afbau principle -orbitals with lower energy get filled first.
- The molecular orbital which is occupied by electrons and has the highest energy is called HIGHEST OCCUPIED MOLECULAR ORBITAL(HOMO) and the orbital with lowest energy which is vacant is termed as LOWEST UNOCCUPIED MOLECULAR ORBITAL(LUMO). The energy difference between them is called HOMO-LUMO gap.

Now let us begin by constructing a MO diagram for H2 molecule.
H2 molecule –
The atomic orbitals (AO) are drawn on the extreme right and extreme left of the MO diagram. There is one electron each in the 1s AO of hydrogen atom. These two AOs form two orbitals –
Bonding orbital (σ) – This is drawn in the centre, between the two AOs and it lies below the two AOs of hydrogen.This is because it has lower energy than the two atomic orbitals.
Anti bonding orbital (σ*) – This orbital again lies in between the two AOs but it is higher in energy than the two AOs and so it lies above them.
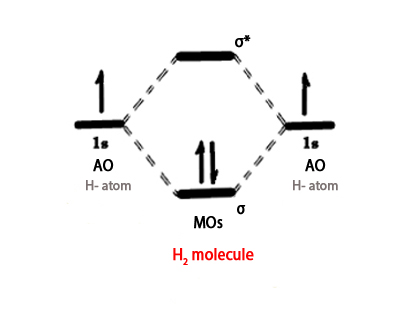
As we know, the electrons shall pair up and then occupy the MO with the least energy (Aufbau principle), which is always the bonding MO. So, the two electrons sit in the BMO(σ1s), as shown in the above figure. Remember, the BMO is delocalized i.e it is spread on both the atoms and so the electrons are not on anyone atom anymore. Thus, it is drawn in between the AOs of two atoms. In this case, the ABMO is vacant.
In the above figure, σ is HOMO, and σ* is LUMO.

∴Bond order = 1/2 ( 2-0) = 1. Thus, there is a single bond between two H- atoms in an H2 molecule. This is experimentally verifiable too .
Electronic Configuration
We know that the electronic configuration of an atom tells us the whereabouts of an electron in it. Similarly, molecules have an electronic configuration too. This configuration gives us a clear picture of the way electrons occupy the MOs.
The electronic configuration of H2 molecule can be represented as – (σ2s)2
Now that we know how to construct the MO diagram, we shall study the symmetry of MOs in the next post. Till then,
Be a perpetual student of life and keep learning...
Good day !