From this post onwards, we shall study some examples and learn how bonds are formed between two different atoms.
Hydrogen fluoride (HF) –
We begin discussing the simplest heteronuclear molecule – the HF molecule. In this species, fluorine is more electronegative than hydrogen. As studied in the earlier post, the 2s orbitals are way too low in energy (-40.2eV) to interact with 1s of hydrogen(-13.6eV).
Why are the 2s orbitals so low in energy?
The atomic number of fluorine is 9 i.e there are 9 protons in a fluorine atom. Thus, the 1s and 2s electrons, which are near the nucleus, experience a very strong pull(more attraction) from these 9 electrons, on account of which, they are very tightly bound to the nucleus. Thus, they are further lower in energy than H electrons, which experience pull from a nucleus that has only 1 electron in it.

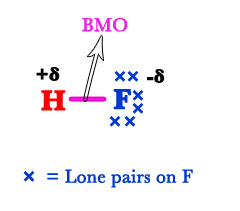
As seen in the figure below, the 2s electrons remain nonbonding (nb).In classical terms, this is a lone pair of electrons, which DOES NOT take part in bonding. As this orbital does not interact with another orbital, its energy remains the same.

The 1s of H atom and 2p orbitals of the F atom are comparable in energy. So, they interact. However, it is only the pz orbital that has the same symmetry around the internuclear axis as the s – orbital. As a result, the 1s of H overlaps with the pz orbital of F atom. The px and py orbitals remain unaffected as they are in a different plane. The electrons in these orbitals are nonbonding i.e they are lone pairs on F atom.

Thus, there are three lone pairs on F atom and the electrons in BMO are shifted more towards F atom too. This is also seen in the Lewis structure of HF molecule. The δ+ charge indicates that the BMO/electron pair is shifted away from H and δ- indicates a partial negative charge that is developed on F atom as a result of the electron pair shifting towards it.
We shall continue studying more MO diagrams in the upcoming posts.
Be a perpetual student of life and keep learning….
Good Day!