With this post, we start discussing MOT theory for diatomic heteronuclear molecules – molecules formed by two different atoms.
The MO diagrams for heteronuclear atoms are slightly different than the homonuclear ones. This is because, in heteronuclear molecules, the contribution from two different atoms is different.
It is imperative that for two different atoms to interact, they must have similar energies and should have the same symmetry characteristics w.r.t the internuclear axis. If these conditions are not met then there can be no overlap between them.
The relative energy of the bonding orbitals determines the magnitude of the bond energy of the molecule, ΔE. The more electronegative element AOs have lower energy than the other atom.
As seen in the figure below, a more electronegative atom has lower energy AO. The bonding MO formed, is just a little lower in energy than this AO and so the net decrease in energy( ΔE) is less.
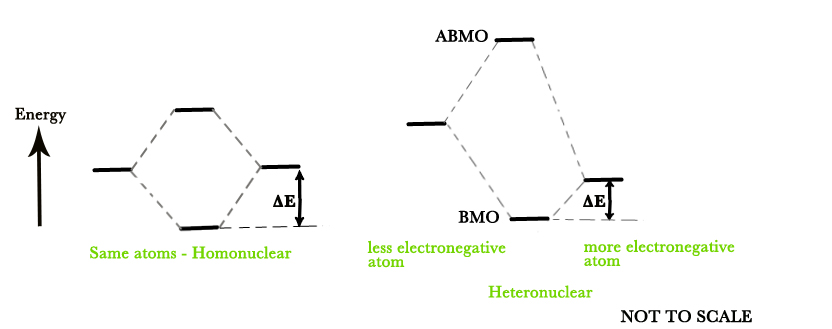
The MOs of heteronuclear atoms are not symmetrical as in the case of homonuclear molecules. The MOs are polarized. The BMOs are shifted towards the more electronegative atom i.e the more electronegative atom attracts the bonding MOs more towards itself. Thus,
the more electronegative atom(nucleophile) contributes more to the BMO→ Mathematically, MOs have a larger coefficient(c) on the more electronegative atoms.
and
the less electronegative element(electrophile) contributes more to the ABMO → MOs having smaller coefficients on less electronegative atom.
Thus, in the above figure, the BMO is drawn closer to the more electronegative element and the ABMO is drawn closer to the less electronegative element.
The bond between homonuclear atoms will always be stronger than the heteronuclear ones owing to perfectly matched AOs.The strength depends on the relative energies of the atomic orbitals participating in bond formation. AOs which are closer in energy will overlap and those with huge energy differences won’t.
|
Type of molecule |
ΔE |
Bond strength |
|
Homonuclear |
ΔE = 0 |
Very strong bond (as AOs are perfectly matched). |
|
Heteronuclear with less energy difference between AOs |
ΔE is less |
Strong bond |
|
Heteronuclear with more energy difference between AOs |
ΔE is more but ΔE < 12eV |
Weak bond |
| Heteronuclear with an energy difference of more than 12 eV |
ΔE > 12eV | No interaction between orbitals |
The following figure shows us relative energies for AOs of different atoms. The data is collected using a technique called photoelectron spectroscopy (We will study this technique in detail in a later post).
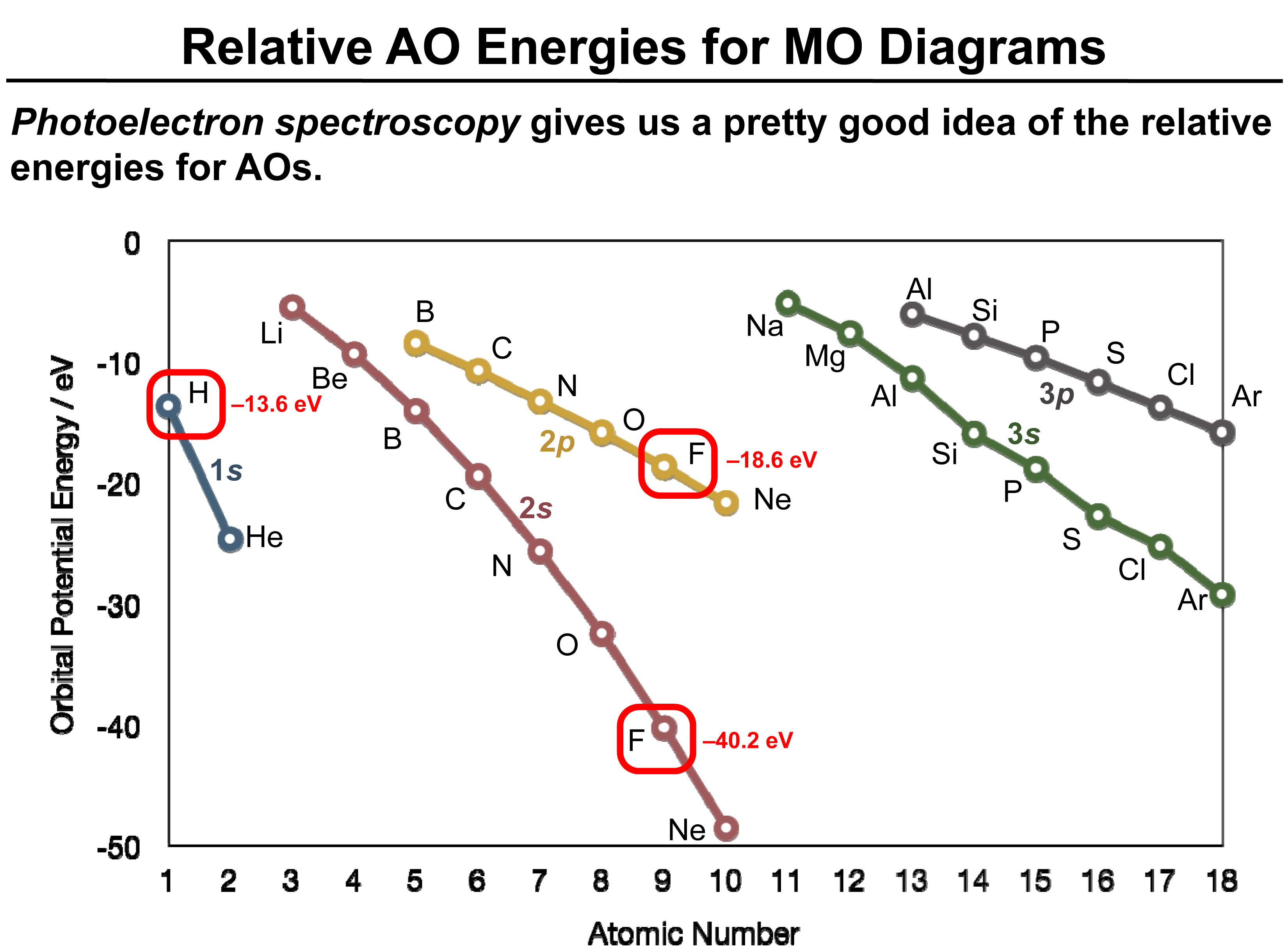
In the above figure, the potential energy(in eV) of a valence orbital is plotted on the Y-axis and the atomic number is plotted on the X-axis.
( We consider kinetic energy for moving objects and potential energy for stationary things. Thus, we talk about the kinetic energy of an electron and the potential energy of an orbital).
We start with 1s orbital for H and He, as they are the valence orbitals for these atoms. Each curve represents a different atomic orbital. As seen in the above figure, the orbital energy of the H atom is – 13.6 eV. This means that it takes -13.6 eV energy to remove the valence (1s) electron out of that orbital.
If the energy difference between two orbitals is greater than 12 eV, the AOs will not interact. Thus, looking at the figure above, we can predict which orbitals can interact with each other and the ones that cannot. As highlighted in the figure, the 1s orbital of H CAN NOT react with the 2s of fluorine atom as –
Energy of 1s of H atom = -13.6ev &
Energy of 2s of F atom = – 40.2 eV.
The energy difference is huge(26.6 eV).
However,
Energy of 2p of F atom = -18.6 eV.
Energy difference = 18.6 -13.6 = 5eV
∴1s of H can interact with 2p of F atom.
(NOTE – the negative sign is not considered while calculating the energy difference ).
Based on this information, we shall begin drawing MO diagrams for heteronuclear species in the next few posts. Till then,
Be a perpetual student of life and keep learning…
Good Day!
Image source –
1.https://www.chem.uci.edu/~lawm/10-9.pdf
References and Further Reading –