We have been reiterating that aromatic compounds have 4n+2π electrons and antiaromatic compounds 4n π electrons. Ever wondered why these numbers are so important? Let’s delve a little deeper and find out why.
What is so special about 4n+2 π electrons?
The answer to this question can be explained by molecular orbital theory. There is a lowest energy MO for every aromatic compound above which are degenerate MOs(MOs having same energy) in the bonding region of the energy level diagram. To fill up these levels , it takes two electrons(one pair of electrons) to fill the energy levels completely. We have already seen how to draw the frost diagrams. A general energy level diagram can be shown as follows –
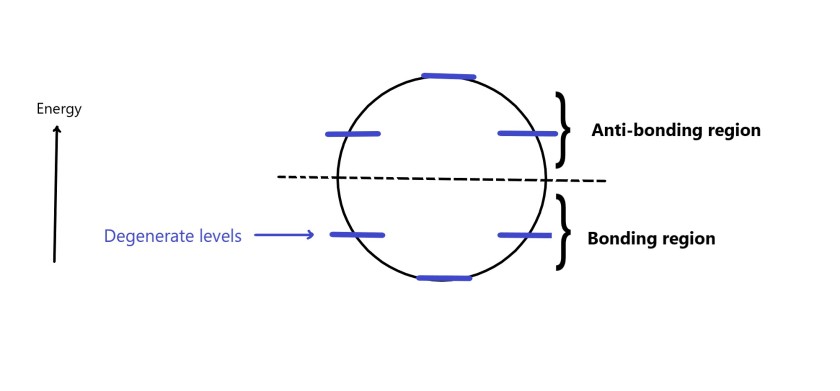
Now lets study MO diagrams with substituting different values of n in (4n+2) formula and see the results-
- If n=1 , we get , 4(1)+2 = 6 electrons.
- If n=2, we get, 4(2)+2 = 10 electrons.
- If n=3, we get, 4(3)+2 = 14 electrons.
 For 6 π electrons ,as in benzene, there are 6 atomic orbitals which will form 6 molecular orbitals namely – ψ1 , ψ2, ψ3,ψ4* ,ψ5* and ψ6*. Three will be bonding and three anti bonding orbitals.
For 6 π electrons ,as in benzene, there are 6 atomic orbitals which will form 6 molecular orbitals namely – ψ1 , ψ2, ψ3,ψ4* ,ψ5* and ψ6*. Three will be bonding and three anti bonding orbitals.
For 10 electrons , we will have 10 MOS –
i)Five bonding from ψ1 to ψ5
ii) Five anti – bonding from ψ6* to ψ10*
For 14 electrons , we will have 7 bonding and 7 anti bonding orbitals as seen above.
As seen in the above figure, with 4n+2 π electrons, all electrons are in the bonding region and are paired.This arrangement of electrons imparts stability to these molecules. Thus, these species are aromatic.
What happens when there are 4n π electrons?
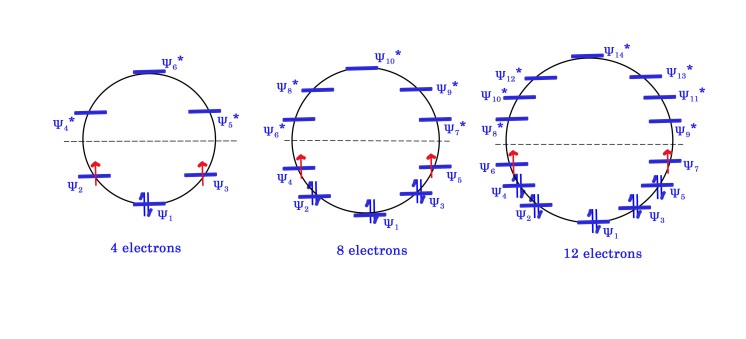
We have considered 4n π electrons in the figure above-
When n=1 , we have 4(1) =4 π electrons.
When n=2, we have 4(2)= 8 electrons.
When n=3, we have 4(3) = 12 electrons.
With all the above numbers , we get a single electron in two orbitals (have highlighted these in red color in the fig). This filling of electrons takes place according to Hund’s rule of maximum multiplicity. This species is called as a diradical. As the electrons are not paired, these diradicals are very unstable and reactive. Thus, such compounds are anti-aromatic.
Six is the Hückel number most often encountered. Why?
To have overlapping p- orbitals , the aromatic ring must be trigonally hybridized (bond angles = 120º) and the structure must be flat. The number of trigonally hybridized atoms that will fit a flat ring without undue strain is 5,6 or 7.Six is the number which satisfies the Hückel’s rule. Thus, 6π aromatic systems are more common.
Thus, with these molecular diagrams, we now understand in better light , why some compounds are more stable and some very unstable. These diagrams help us understand why 4n+2 π electrons is a pre-requisite to an species being aromatic. In the next post we will see in detail, the different criteria required for a species to be aromatic.
Till then,
Be a perpetual student of life and keep learning….
Good Day !