In the last post we left some questions unanswered. Questions like –
How does one relate the number of particles to volume and mass of the object?
What is Avogadro’s hypothesis?
Let us find answers to these questions in this post.
Avogadro’s hypothesis
After studying Gay Lussac’s law of combining volumes of gases, Amedeo Avogadro put forth his own hypothesis , which stated –
“Equal volumes of all gases under the same conditions of temperature and pressure contain the same number of molecules.
or
Equal number of molecules of all gases occupy the same volume under the same conditions of temperature and pressure.“

At STP conditions (STP = standard temperature and pressure – 0°C and 1 bar pressure) ,

One mole of any gas occupies 22.4 dm3 i.e 22.4 litres volume.
∴22.4 litres of any gas at STP has 6.023 × 1023 molecules of that gas.
Volume of 1 mole of any gas is called its MOLAR VOLUME.
e.g.– If we take 22.4 litres of nitrogen gas in a container, it will contain 6.023 × 1023 molecules of N2 gas.

If the amount of gas taken increases then its volume increases too and if the amount of gas decreases, the volume is going to decrease subsequently. Thus, we can say that ,
V α n where,
V⇒ Volume of the gas
n ⇒ no.of moles.
Introducing a proportionality constant , we get ,
V = k n. (k = constant of proportionality).
∴V/n = k.
Thus, we can state, (V1 / n1 ) = (V2/n2).

Mathematical expression for Avagadro’s law – (V1 / n1 ) = (V2/n2).
Now isn’t this obvious? Consider a balloon. When you fill in more air , the balloon will grow in size.
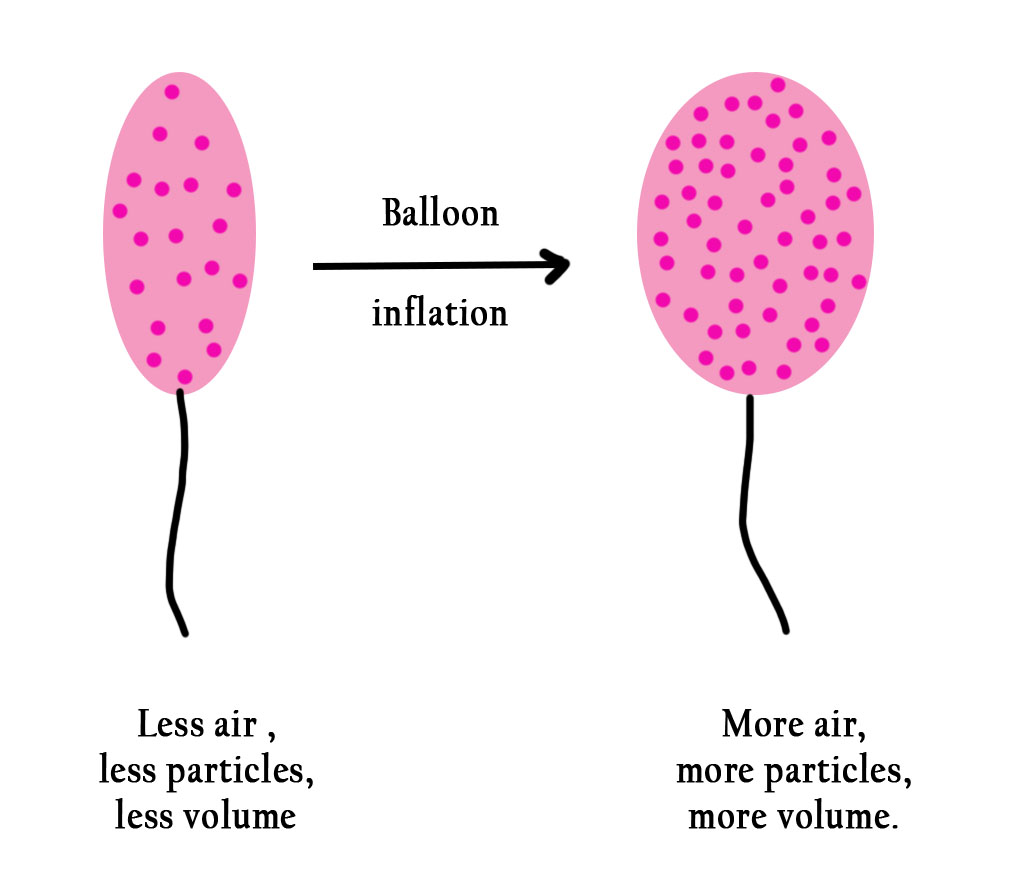
How to explain this in a chemical reaction?

In the above reaction, if 1 mole (or 22.4 lit) of hydrogen is used to react with 1 mole of chlorine, then 2 moles(44.8 lit) of hydrogen chloride gas is formed. If the amount of reactants is doubled, the volume of product is also doubled.
The ratio of volumes of reactant and product gases remains constant – 1:1:2.
Problem – Calculate the number of molecules in 5.6dm3 of CO2 at STP.
Solution –
22.4 dm3 of CO2 at STP contains 1 mole of that gas.
∴ 5.6 dm3 of CO2 will contain (5.6 /22.4) = 0.25 moles of the gas.
Also , 1 mole = 6.023 × 1023 molecules.
∴ 0.25 moles will have 0.25 ×6.023 × 1023 = 1.506 × 1023 molecules.
Thus, Avogadro’s hypothesis helps us to relate the number of particles ( in moles) and volume of gases. In the next post we shall see how we can relate the mole to another measurable quantity – mass. Till then ,
Be a perpetual student of life and keep learning….
Good day !