In this post, we are going to study one of the most seminal concepts, not only in analytical but in entire chemistry. Understanding this concept is crucial to understanding the quantitative relationship between reactants and products. However, before we proceed any further , we need to first understand the difference between mass and weight of a substance.
The difference between Mass and weight
In day to day conversations we use both these terms interchangeably. However, scientifically these two terms are different. In post 43, we have already seen this difference. Let us again revise it here.
Mass (m) ⇒ Amount of matter in the substance.
Weight ⇒ Amount of gravitational pull on that mass.
![]()
w= m × g , where g is the acceleration due to gravity.

As seen in the figure above, the mass of an object doesn’t change according to its location. However, its weight does. The gravitational pull exerted by the earth is different in different places and thus the weight varies.

This is a picture of astronaut Edwin Aldrin on the moon. The space suit he is wearing is very heavy.However , the acceleration due to gravity on moon is 1/6th time of that on earth, the suit weighs six times less on the moon (although its mass remains the same !).
A chemical analysis is always based on mass and thus the results will not vary with the locality. Thus, although we say that we weigh objects or we talk about weights , in the literal sense , we are only considering the MASS.A balance is used to compare the mass of an object with standard masses in a region.
After understanding the difference between weight and mass , let us now begin our discussion on the mole concept, which helps us define amount of a substance.
The Mole Concept
The term ‘mole’ was coined in 1896 by a Noble prize winner chemist Wilhelm Ostwald. He derived this term from Latin word moles meaning ‘a heap’ or ‘pile’.
Atoms and molecules are really very small, so we cannot count them individually. Thus, the mole concept was formulated to be able to express the number of atoms/molecules/ions/particles as a cluster or group.
1 mole = 6.023 × 10 23 units .
6.023 × 10 23 is called as the Avogadro’s number.
Just as we know ,
1 dozen = 12 units,
1 mole of anything = 6.023 × 10 23 units of that thing.

However,the Avagadro’s number refers to a huge number of particles –
6.023 × 10 23 = 60230000000000000000000 entities !!!
How large is this number ? If 6.023 × 10 23 pieces of paper are stacked together, the stack would reach beyond our solar system !

Can we count so many units of anything everytime we make measurements? The answer is obviously NO. So, we have to relate this number to measurable quantities like mass or volume.

How was the relationship between number of molecules and volume established ?

Amedeo Avogadro was an Italian physicist , who started studying the Gay Lussac’s law.Sir Gay Lussac (1778-1850), was a French chemist.He stated a law for combining volumes of gases, which stated-
“There exists a simple relation between the volumes of gases and their atoms/molecules.
When gaseous reactants react to give gaseous products ,the volumes of the reactants and products bear a simple whole number ratio to one another(provided the volume measurement is carried out at constant temperature and presuure).
In short , the ratios of volumes of reactant and product gases are small whole numbers.The ratio will never have integers/decimals or fractions.
e.g.– 1 volume of nitrogen gas combines with 3 volumes of hydrogen gas to form 2 volumes of ammonia gas. Thus, the ratio of volumes of gaseous reactants and products is 1:3:2. This is a simple whole number ratio.
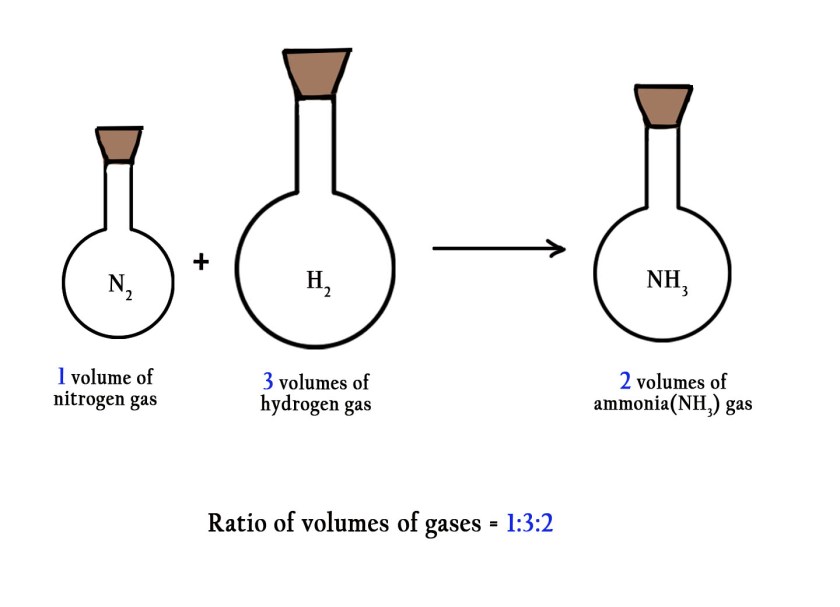
Avogadro concluded that if gases are made up of molecules and gases combine in simple whole number ratios of their volumes, then there has to be some relation between the no. of molecules present in the gas and the volume of that gas. He thus put forward Avogadro’s hypothesis relating volume of gases and number of molecules.
What was that hypothesis? How does that hypothesis relate to the mole concept? We will find that out in our next post. Till then ,
Be a perpetual student of life and keep learning….
Good day !
Image source –
1.http://www.clipartpanda.com/categories/planet-earth-clipart
2. https://www.thefamouspeople.com/profiles/amadeo-avogadro-532.php
3.https://wallpapercave.com/w/cfQ9vvX
4.https://clipground.com/people-man-scared-clipart.html
5.https://www.pinclipart.com/pindetail/iRJmxR_clip-art-black-and-white-download-business-man/
6.http://clipart-library.com/clipart/solar-system-clipart-15.htm
