From this post onwards, we shall begin our discussion on a new topic in physical chemistry – Photochemistry.
What comes to your mind when we say the word ‘photo‘? We use this word many times in our day to day lives e.g.– photography, photosynthesis , photoshop , photoshoot etc. What do all these words have in common? ‘LIGHT‘!! So then what is light? Something that lits up things or removes darkness? Scientifically, light is a part of electromagnetic radiation that is visible to human eye.
What is electromagnetic radiation? What exactly is light? What are photons? How does this light bring about chemical reactions? What are different kinds of photochemical reactions? We are going to answer all these questions in the upcoming posts on photochemistry.
ELECTROMAGNETIC RADIATION
Radiation means ‘rays’ or ‘waves’. Waves are vibrations having crests and troughs. As seen in the figure below, crests are above the equilibrium position and troughs are below that position.
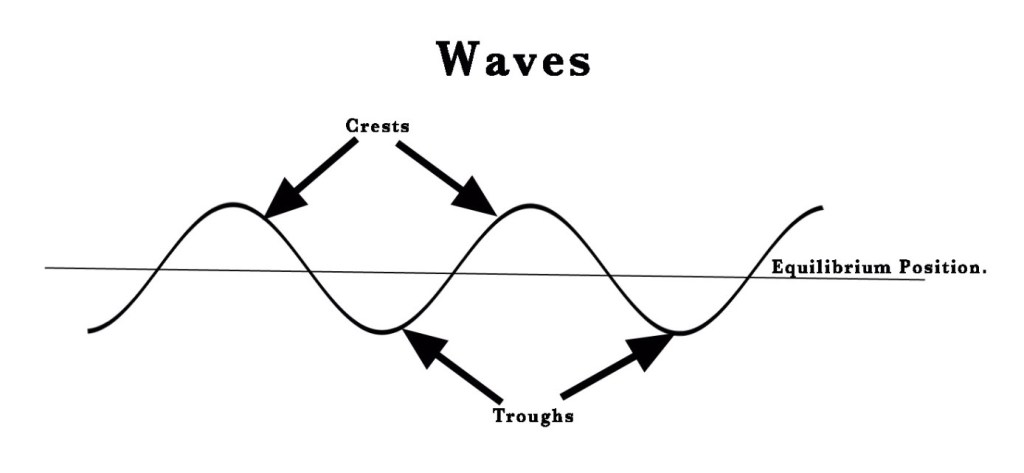
Radiation is the form of energy that is transmitted or emitted in the form of waves or particles (Read about waves and wave- particle duality from post #30) .
e.g.– Solar radiation means the energy that comes from the sun. Just go out on a hot sunny day and feel the heat! This is heat energy from the sun.We get light from the sun – light energy!

What is electromagnetic radiation? As the name suggests , radiation that has both electro (electrical) and magnetic components is electromagnetic radiation.Both these components are perpendicular to each other as seen below –

Thus,electromagnetic radiation is an electric and magnetic disturbance propagating through space. This radiation travels at the speed of light (3 × 108 m/s). It contains neither mass nor charge but travels in packets of radiant energy called photons, or quanta.
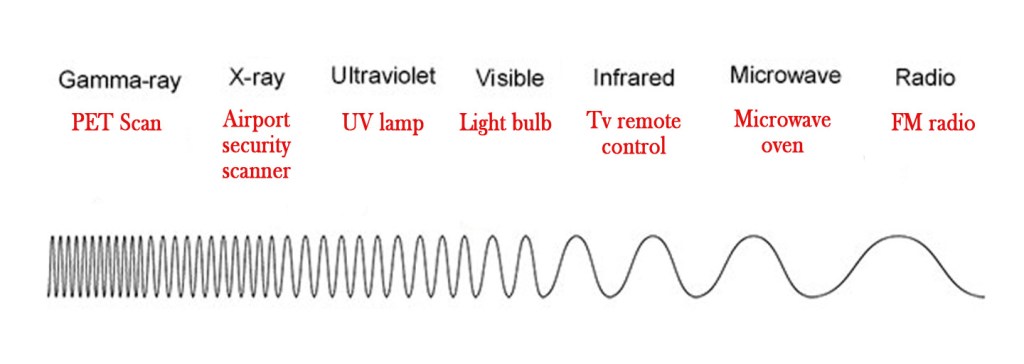
The light that we see is just part of the electromagnetic radiation spectrum.This spectrum has waves with different wavelengths.The electromagnetic spectrum can be shown as below –

As seen in the above figure, visible range is only a small part of the spectrum.There are many kinds of waves – Gamma rays,X-rays ,IR rays,UV rays etc. They all have different wavelengths and different properties.Depending on their respective characteristics, these rays are used widely in our day to day lives as follows-
PHOTONS
As studied in post #30, wave-particle duality states that, waves can be modelled as particles too.The particles of light are called as ‘PHOTONS‘. Photons are the smallest unit/quanta of EM radiation. So, basically light is the flow of photons.These photons are constantly in motion and have zero mass.They have no electrical charge.
Now that we know what EM radiation is, we shall proceed to study how this radiation brings about various chemical reactions.The next post will be an introduction to photochemistry.
Be a perpetual student of life and keep learning…
Good day !
Image source –
1.https://www.webmd.com/a-to-z-guides/ss/slideshow-sunlight-health-effects
2.https://imagine.gsfc.nasa.gov/science/toolbox/emspectrum1.html