Absorbance and Transmittance
Absorbance(A) and transmittance(T) are two sides of a coin. Absorbance (A) is the amount of light absorbed and transmittance is the the amount of light transmitted by a solution (after it passes through the solution). The intensity of the incident light beam (Io) is always greater than or equal to the intensity of transmitted light (It).

To understand what this means we can take the following values for both terms –
| Absorbance(A) | Transmittance(%T) |
| 100% | 0% |
| 0% | 100% |
Thus, if a solution absorbs all the light incident upon it (100%) then ,no light will be transmitted (0% T). Correspondingly, if the solution does not absorb any light then it will transmit all the incident light. Thus, the absorbance will be 0% and transmittance 100% .
Transmittance(T)
Consider monochromatic light with incident intensity I0 ,which passes through a solution. The beam which comes out after it has passed through the solution is called the transmitted beam. The transmitted light intensity is given by It.

The transmittance (T) is the ratio of the intensity of the transmitted light to the intensity of the incident light. As this is a ratio, it does not have any units. Transmittance takes values between 0 and 1 but usually transmittance is expressed as %T (percentage transmittance) –
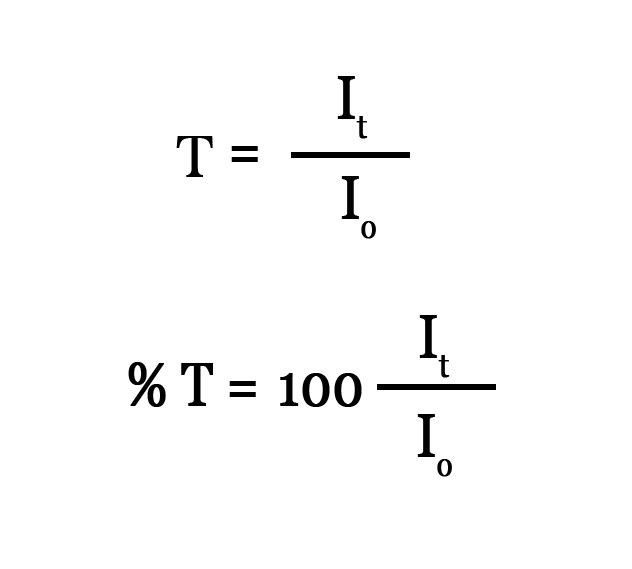
Why does transmittance take values between 0 and 1 ?
Consider the following three cases –
CASE 1 – Some of the incident light is absorbed by the solution.

When the solution absorbs light ,the intensity of incident light will be more than transmitted light – Io >> It –
T = It / I0 << 1
Thus, the transmittance T has values less than 1.This means that light is absorbed by the solution. Only a fraction of incident light little is transmitted (Thus, transmittance is less).
CASE 2 – All of the incident light is absorbed by the solution.
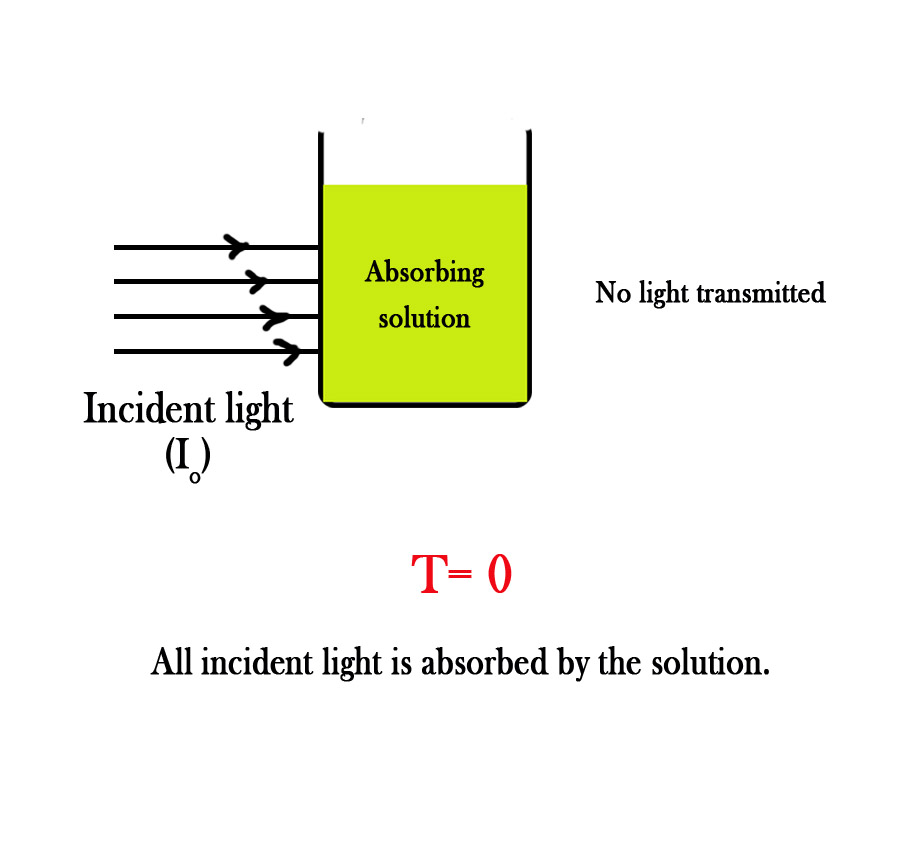
If the solution absorbs all the light incident upon it, no light will be transmitted and thus transmittance will be zero .
T = 0 / I0 = 0
CASE 3 – Incident light is NOT absorbed by the solution.

If the solution does not absorb any light (non-absorbing solution), it will transmit all the incident light. Thus, I0=It . In this case –
T = It / I0 = 1
The transmittance will be one – which means all the incident light is transmitted and no light is absorbed.

It can NEVER be more than Io. The transmitted light cannot be more than the light incident on the solution.
In the next post we will talk about absorbance in detail. Till then,
Be a perpetual student of life and keep learning…
Good day !