The Lambert’s Law
We studied in the previous post, that according to Lambert’s law, absorbance (A) is directly proportional to the path length or thickness (b) –
A ∝ b

In the above figure, the cell containing the absorbing solution in case 2 has a greater path length and so more light is absorbed, than in case 1( the concentration of both solutions is the same). This means that each successive layer of the medium absorbs an equal amount of light and thus as the path length(b) increases, more and more light gets absorbed. Thus, the intensity of the transmitted beam is reduced.
The mathematical expression for lambert’s law is given as follows –


August Beer was a German Chemist, physicist, and mathematician. He was studying the absorption of red light by various solutions when he formulated a law called Beer’s law.
The quantity of light absorbed by a solute (dissolved in a non absorbing solvent) is directly proportional to the concentration of the solute.
Beer’s Law
or
The absorptive capacity of a dissolved substance is directly proportional to its concentration in a solution.
According to Beer’s law, absorbance (A) is directly proportional to the concentration (c)
A ∝ c

As is obvious from the figure above, the more concentrated solution in case 2 will absorb more light than the less concentrated solution in case 1(even though the path lengths are the same).
The mathematical expression of Beer’s law is similar to that of Lambert’s law –
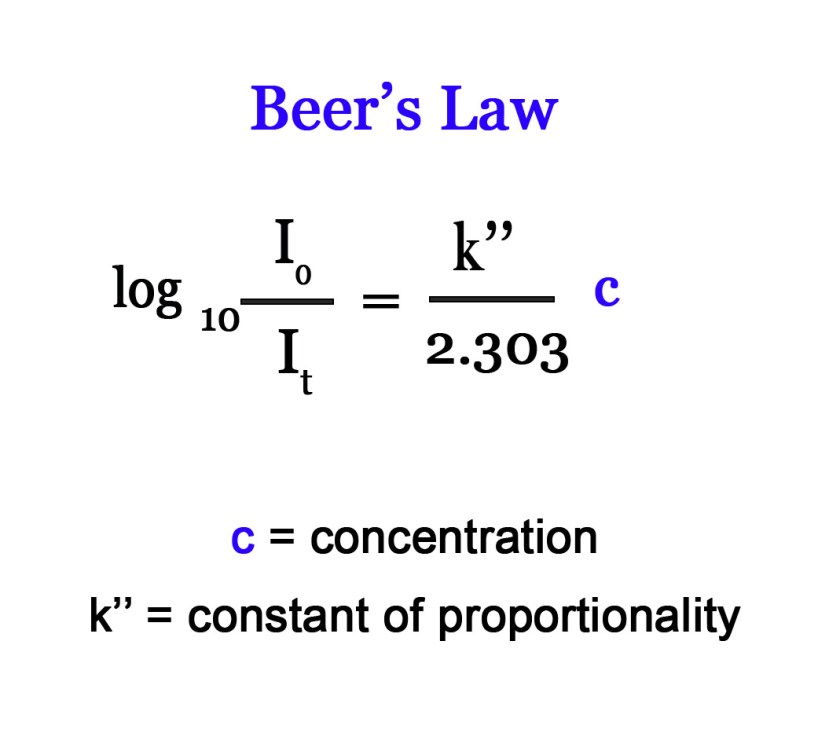
k” is another constant.
A helpful video on Beer’s law – https://youtu.be/4GI-6uR8k4o
Beer -Lambert’s law
The Lambert and Beer’s law put together gives us the Lambert-Beer/Beer-Lambert’s law which can be stated as follows –
The quantity of light absorbed by a solution is directly proportional to the concentration of the solute and the path length of the light through the solution.
Beer – Lamberts’ law /Lambert – Beer law.
A ∝ c b
Introducing the constant of proportionality(a) , mathematically, Beer- Lambert’s law can be written as –
A = abc
where,
A = Absorbance of the solution ( No units )
b = thickness /path length ( cm )
c= concentration of the solution i.e amount of solute in the solution ( g/dm3)
a = Absorptivity ( cm-1 g-1 dm3 ).

The thickness is always in cm. However, the concentration(c) can be in g/dm3 or mol/dm3. If the concentration is expressed in g/dm3 then, the constant of proportionality is absorptivity (a), as shown above. If the concentration is expressed in mol/dm3, the constant of proportionality is referred to as molar absorptivity ( ε ) as shown below.
A = εbc
where,
A = Absorbance of the solution ( No units )
b = thickness /path length ( cm )
c= concentration of the solution i.e amount of solute in the solution ( g/dm3)
ε = Molar absorptivity (cm-1 mol-1 dm3 ).
Combining the two exponential expressions of Beer’s and Lambert’s law, we get –
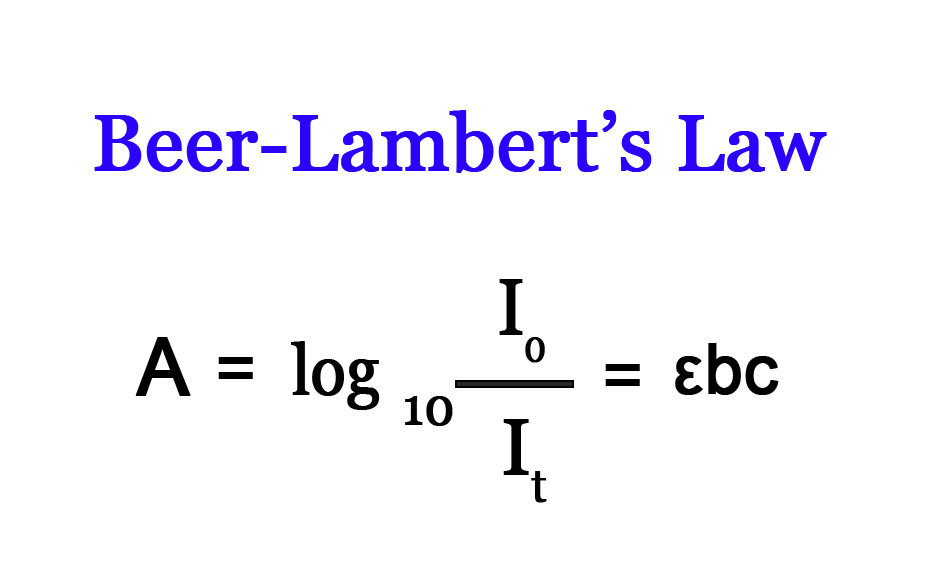
How is this law helpful?
This law helps us to calculate the concentration of the unknown solution. How?
We take the unknown solution and pass light through it. This is the incident light. We know the intensity of this incident light (I0). After passing light from the medium, we record the intensity of the transmitted light(It). We measure the thickness of the medium (b). ε is a constant and thus we know its value. If we plug all these values in the above expression, we can easily find the concentration of the solution(c)! The device used to measure the intensity of transmitted light is called a spectrophotometer. To know more about spectrophotometers read post 161.
Thus, if we calculate the absorbance (A = Io/It) of the solution experimentally, then we can easily calculate the concentration of the absorbing species in the solution. Thus, this law helps us to determine the concentration of solutes in a solution.
Intensity of light
Before we proceed any further, it is important that we understand the concept of the intensity of light. What does intensity mean? In the afternoon we say ‘the sun is very intense‘ because the intensity of light is high. During sunset though, that intensity reduces and thus, we can look at the sun directly.
Thus, intensity is the quantity of radiation/ no. of photons emitted per unit of time. The everyday term for it is ‘brightness’. The more the brightness of light, the more is its intensity, and vice versa.

In the next post, we shall study what absorbance and transmittance mean and further talk about the laws of photochemistry. Till then,
Be a perpetual student of life and keep learning…
Good day!
One comment