In the previous post we studied the basic components of a spectrophotometer. Now let us learn each component in a little more detail.
Light source (LS)
A light source for a spectrophotometer is expected to emit light in the UV, visible or IR range –
| Radiation | Wavelength in nm |
| UV – C (short wavelength) | 100-280 |
| UV – B (medium wavelength) | 280-315 |
| UV – A (long wavelength) | 315-400 |
| Visible | 400-800 |
| Near IR | 800-3000 |
| Mid IR | 3000-20000 |
| Far IR | 20000-300000 |
The general requirements for a good light source are –
1) has to be stable over time.
2) has to be bright across a wide range of wavelength
3) uniform brightness
4) should have a long service life
The light sources generally used in spectrophotometers are –
Halogen / Tungsten /Tungsten – Halogen Lamp –
This is an incandescent light source. Incandescent means a light source with a wire filament in it. When electricity is supplied to this wire, it gets heated and emits light.

Tungsten is a metal with the highest melting point – 3422 ° C .Thus, this element is a great choice for making the filament. As seen in the above figure, the filament is not straight but a coiled wire. This increases the surface area of the coil and produces more light besides the internal reflections within the bulb.
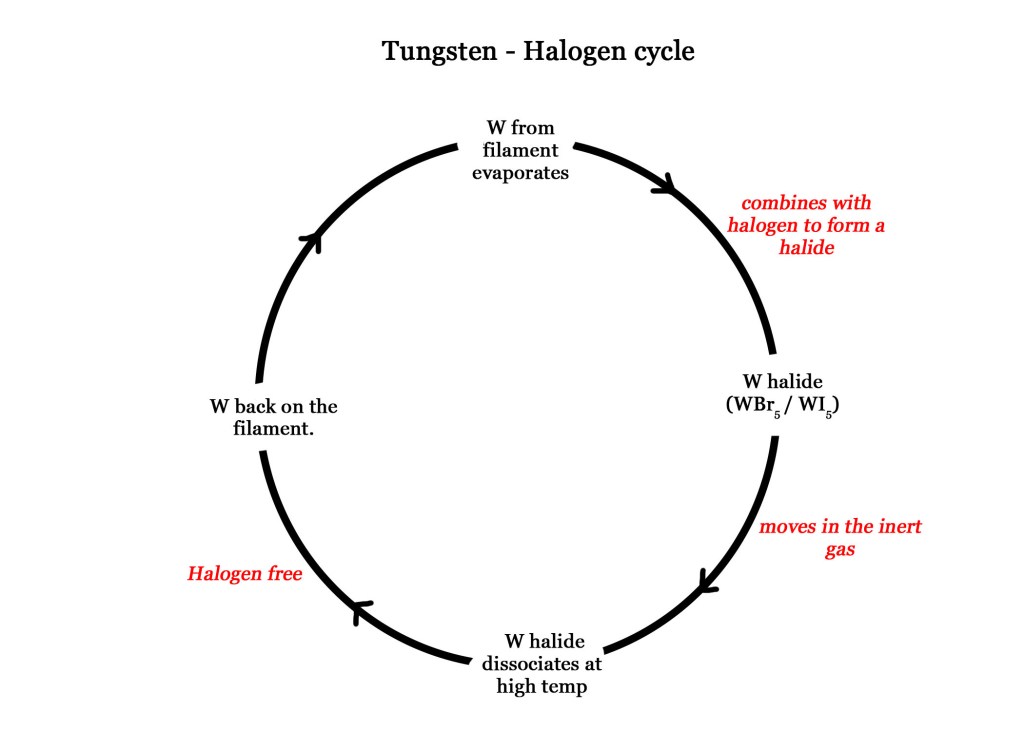
In a normal tungsten bulb, the tungsten atoms evaporate from the filament at high temperatures and deposit on the inner side of the bulb, making it black. Thus, the wire reduces in thickness and eventually breaks ! To avoid this , the bulb is filled with an inert gas and a halide(bromide or iodide).These help in getting the evaporated tungsten back to the filament .
These lamps produce light of wavelengths from near UV to IR region.
Deuterium lamp –
Deuterium (D or 2 H) is an isotope of hydrogen. it is also called as heavy hydrogen. This is a gas discharge lamp , which uses deuterium gas.
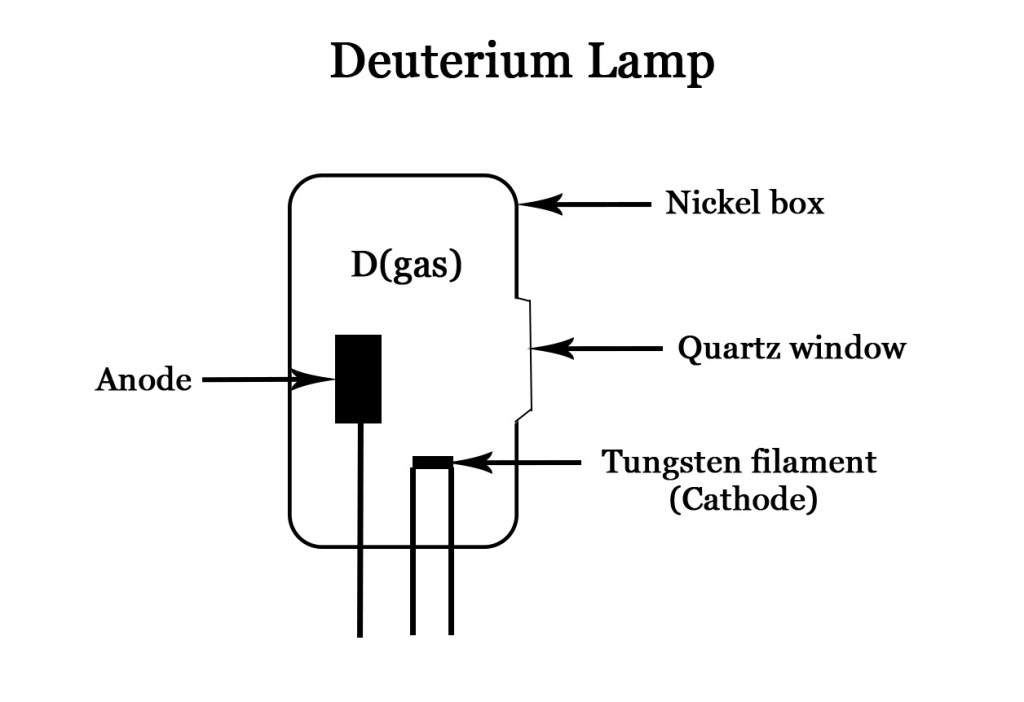
Gases are generally poor conductors but when a suitable ignition voltage is applied, the gas ionises and emits electromagnetic radiation. A typical deuterium lamp looks as follows –
As seen in the figure , in general, a deuterium lamp consists of low pressure deuterium gas, in a nickel box with a quartz window (the design may vary ).High voltage can be applied to the gas , using the cathode and the anode. Note that , even though the cathode is made of tungsten filament, it is NOT the filament that produces light in this lamp. The ionization of deuterium gas leads to emission of electromagnetic radiation. Deuterium produces more intense light than its isotope hydrogen and thus is a preferred choice for this lamp.
This lamp produces light in the UV region.
In the next we shall continue to explore more light sources used in spectrophotometers. Till then,
Be a perpetual student of life and keep learning….
Good day !
Image source – 1.https://simple.wikipedia.org/wiki/Electrical_filament#/media/File:Filament_(PSF).png (bulb with the filament)
References and further reading –
1.https://www.shimadzu.com/an/service-support/technical-support/analysis-basics/fundamentals-uv/lightsources.html#1