Xenon Lamp –
This is a gas discharge lamp where electricity is passed through ionized gas (xenon) at high pressure , to produce light. Gas discharge lamps typically use noble gases, as they are chemically inert otherwise. Different noble gases produce different colours. Xenon lamps are perfect for use in a spectrophotometer.

A typical xenon arc lamp is made up of a quartz gas discharge tube with a anode and cathode at two ends as shown in the figure above. Xenon gas is filled inside the tube. A very high voltage is applied across the two electrodes. Noble gases are poor conductors of electricity , however , when a suitable high ignition voltage is applied across the electrodes, the gas ionizes ( ions are formed).
What happens when we apply high voltage?
The high voltage applied , provides energy to the electrons, in atoms of xenon gas , to get excited to higher energy levels. When these excited electrons fall back to their original electronic levels, they emit energy in form of radiation(light). Thus, they produce light. The energy difference between two electronic levels determines the wavelength of the light produced.

Xenon lamps produce radiation which is in UV , visible and IR range – 185nm to 200nm.However, they give high intensity peaks around 850 – 900 nm.
The envelope of this lamp is made of quartz as it is the only economically feasible material that can withstand high pressure and temperature and is also optically clear. This lamp uses thoriated tungsten electrodes i.e thorium is used as a dopant in them. Dopant is an impurity introduced in the main metal in very small quantities. So, in this case , a little thorium is added to tungsten. How does this help? Addition of thorium enhances the electron emission characteristics of the electrodes.
Collimator lens (CL)
A collimator lens is a device used to get parallel beams of light, as shown in the figure below. This device typically consists of a tube with one or more convex lenses.It is specifically designed to bend light in a way that it produces parallel beams.

Filter (F)
An optical filter is a device which selectively transmits light of specific wavelength/s. These are also called monochromators ( these two are slightly different from each other, but for purpose of our discussion we can use these terms synonymously).
Mono = ONE , chroma = a term related to purity of light. Thus, monochromator means a device which produces one or a narrow range of wavelengths. Having specific wavelength of light is crucial to measurements in a spectrophotometer. Thus, a filter ensures that only light of specific wavelength enters the cuvette , which holds the sample.

Slit (S)
This is a narrow opening , which controls the amount of light entering the cuvette.
Cuvette (C)
This is a tube like , clear container with straight sides , which holds the sample under study. Generally , cuvettes are made of plastic , glass or quartz. The material of the cuvette should be non-reactive and it should be transparent to the wavelength of light used for measurements.
Detector (D)
A detector is a device which converts the transmitted light into proper electrical signals by photoelectric conversion. These are made of photomultilpier tubes(PMT) , which have a photosensitive material in them. As light strikes them , electrons are emitted through the surface which help in giving the signal. The signal tells us the intensity of the transmitted light(It).
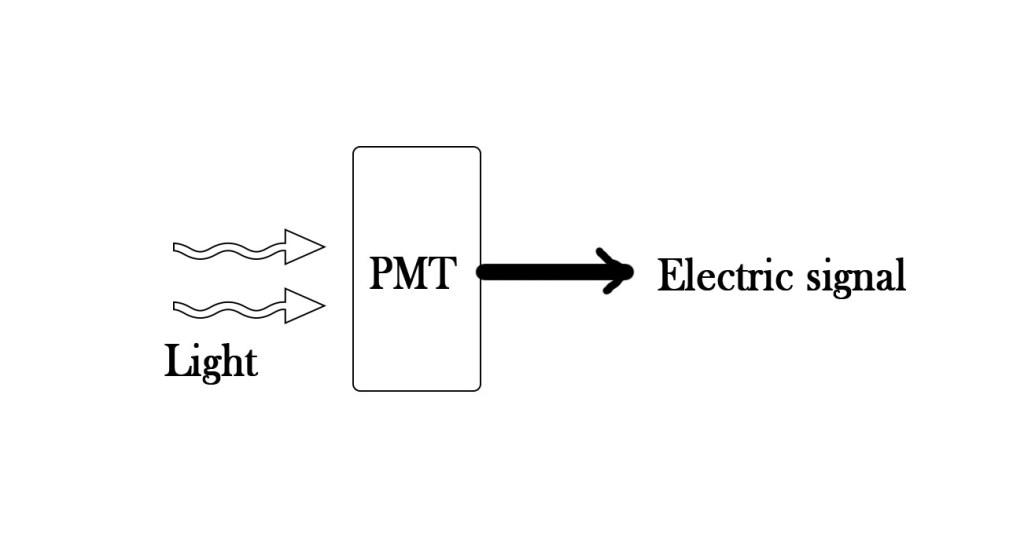
So this is how we measure Io and It to find out the concentration of the sample solution using Beer – Lambert’s law. We have been discussing Beer – Lambert’s law for so long now! Does the discussion end with this post? NO ! In the next post we talk about something more in relation to this law. Till then ,
Be a perpetual student of life and keep learning…..
Good Day !