Understanding the molecular vibrations is very interesting. To locate the molecule in space, we need to predict its behavior. When we try to find the probability of finding the atoms in a molecule, we need to consider the molecule’s vibrations. Let us understand this concept with a simple diagram –
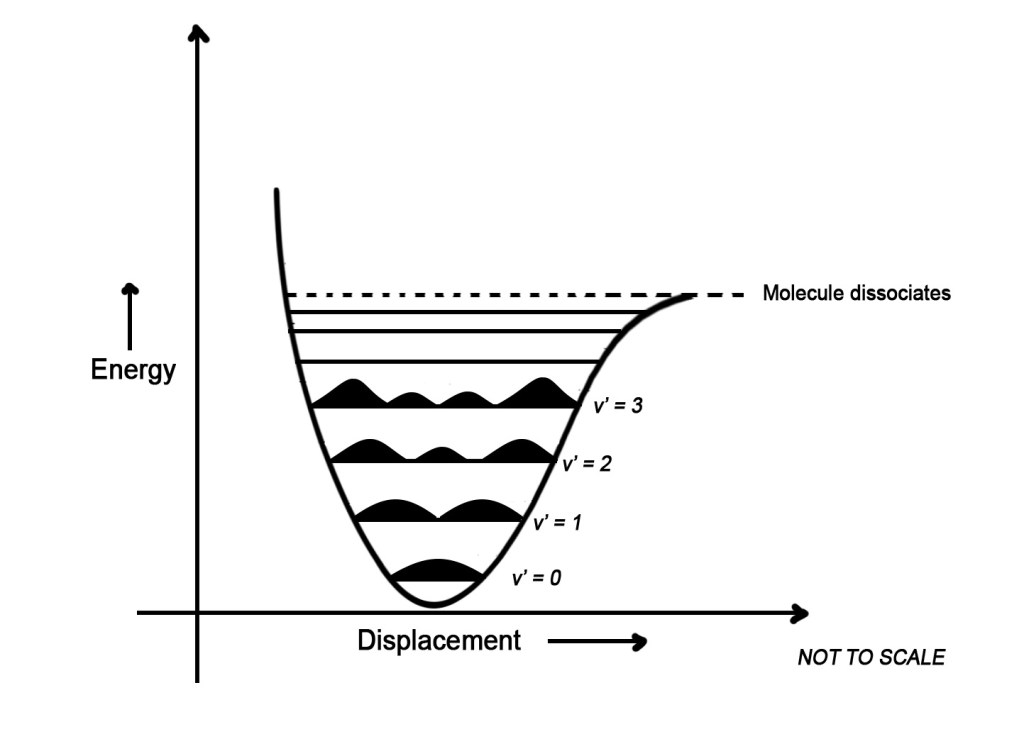
- The above figure is showing us the probability of finding the molecule in a specific region. As seen above, in the ground state (v’ = 0), the molecule is vibrating in an equilibrium position. Thus, the molecule is more likely to be found in the center. Why the center? It is because, when the molecule vibrates and goes to the corners (away from the center), a restorative force pulls it back to the center to maintain the equilibrium. Thus, the molecule spends more time in the center than in the corners. Thus, we see that the probability of finding the electron is more in the center (shown by a black peak, with more height, in the center).
- As we move up, the molecule gains energy and starts vibrating with greater displacement. Now the molecule spends more time in the corners and less time in the center. This is because, though the restorative force is still acting on the molecule, the molecule has more energy to move apart i.e to vibrate more. Also, as the displacement increases, the restorative force also increases. Thus, the molecule is pulled back to the center in less time. Then again it goes back to the corners. Thus, it spends more time in the corners than it would in the ground state.In simple words, as the energy increases,, the probability of finding the molecule is MORE IN THE CORNERS/EXTREME END, than in the center.
This is correctly depicted in the figure above. The peaks at the corners are bigger than the ones in the center. This means that the probability of finding the molecule in the corners is more than in the center.
An important point to note during this discussion is that we are strictly talking about the vibrational energy levels and NOT the electronic energy levels. When a molecule is exposed to radiations in the IR range, it affects the vibrational levels only. This energy is not sufficient to excite a molecule electronically. Thus, there are no electronic transitions in the IR range.
However, the energy in the UV-visible range is sufficient to excite a molecule electronically. Thus, when a molecule is exposed to UV-visible radiation, the electron/s in it get excited to higher energy levels.
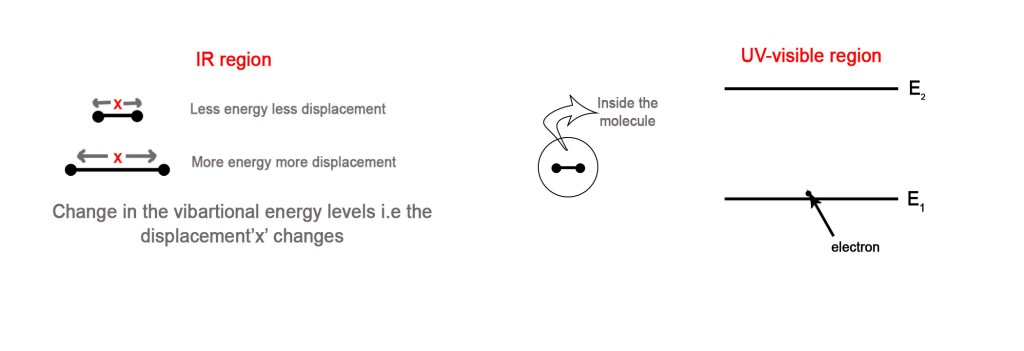
As seen in the above figure, the molecule undergoes changes in the vibrational energy levels, in the IR region. The electrons which are inside the molecule are unaffected. However, in the UV-visible region, the electrons inside the molecule also get excited to higher energy levels. In this case, the electron in the ground state, E1, will absorb radiation and get excited to E2.
Now we ask a very important question,
DOES CHANGE IN THE ELECTRONIC LEVELS BRING ABOUT CHANGE IN THE VIBRATIONAL ENERGY LEVELS TOO?
We will try to find the answer to this question in our next post. Till then,
Be a perpetual student of life and keep learning…
Good Day!