In the previous post, we wondered – DOES CHANGE IN THE ELECTRONIC LEVELS BRING ABOUT CHANGE IN THE VIBRATIONAL ENERGY LEVELS TOO? In this post, we shall answer this question.
The answer to this question is YES. When a molecule gets electronically excited, there is a change in its vibrational energy levels too. We know from post 72, that every electronic energy has numerous vibrational energy levels in it. The following figure shows two electronic levels with their vibrational energy levels, E0 and E1 –
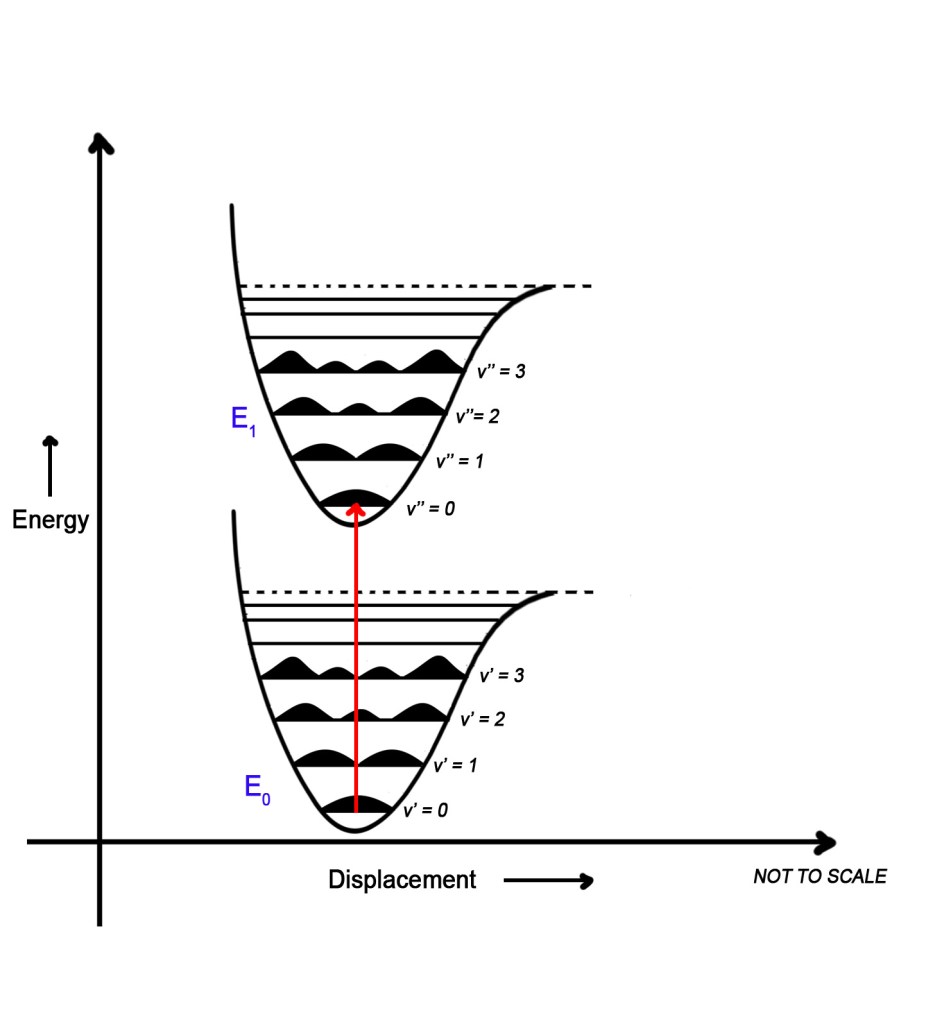
The lower electronic energy level (E0) has less energy and thus is more stable. This is the ground state. The upper electronic energy level (E1) has comparatively more energy. Thus, that is the excited state. Each electronic energy level has corresponding vibrational energy levels in it. The vibrational levels in E0 are labeled as v’ (v prime)and those in E1 as v”(v double prime).
The electron is in the ground state, E1. It can be in any vibrational level. When it absorbs UV-visible radiation, it gets excited and goes from E0 to E1. This electron can go from any vibrational level in E1 to any excited vibrational energy level in E2. Thus, it can go from v’=0 to v”= 3 or from v’=4 to v”= 0.Thus, any such transitions are possible. This means that the probability of all transitions is equal. Thus, the peaks that show us this probability should be equal too. However, we do not observe such equal peaks. Some peaks are more intense than others. So, why do we see this behavior?
This behavior can be explained by the FRANCK-CONDON PRINCIPLE. Before stating the principle, let us first remember that molecular vibrations involve the movement of the nuclei of the atoms. When the atoms in the molecule vibrate, the atoms move close to each other and then move farther apart. There is a change in the internuclear distance and the nuclear configuration in the molecules changes. Nuclear configuration refers to the position of the nuclei of the atoms relative to each other. Thus, when the atoms move apart, the nuclei also move away from each other. Similarly, when the atoms move close, the nuclei also come closer.

The Franck -Codon principle states that,
When a molecule is undergoing an electronic transition, there is no significant change in the nuclear configuration of the molecule.
Franck-Codon Principle
The electronic transitions happen very fast as compared to the vibrations in the molecule. Thus, during the course of an electronic transition, there is no change in the vibrational state of the molecule. The internuclear distance between the molecules DOES NOT CHANGE DURING AN ELECTRONIC TRANSITION.
This is due to the fact that nuclei are very massive when compared to an electron.
Mass of the nucleus ≈ 1.67 × 10 -27 kg
Mass of an electron ≈ 9.11 × 10 -31 kg
Thus, if the nucleus is like an elephant, the electron is like a small fly. And a fly can move much more faster than an elephant. So by the time the fly moves and comes back to its original position, the elephant has not moved at all. Likewise, the electron gets excited and then falls back to the ground state. This happens so fast that in that time frame there is no change in the internuclear distance of the molecule. The electronic transition happens in a time frame so short, compared to the nuclear motion, that the transition probability can be calculated at a fixed nuclear position (fixed internuclear distance). Thus, while plotting the graph, the internuclear distance for both the ground and excited electronic states is the same as seen in the figure below-
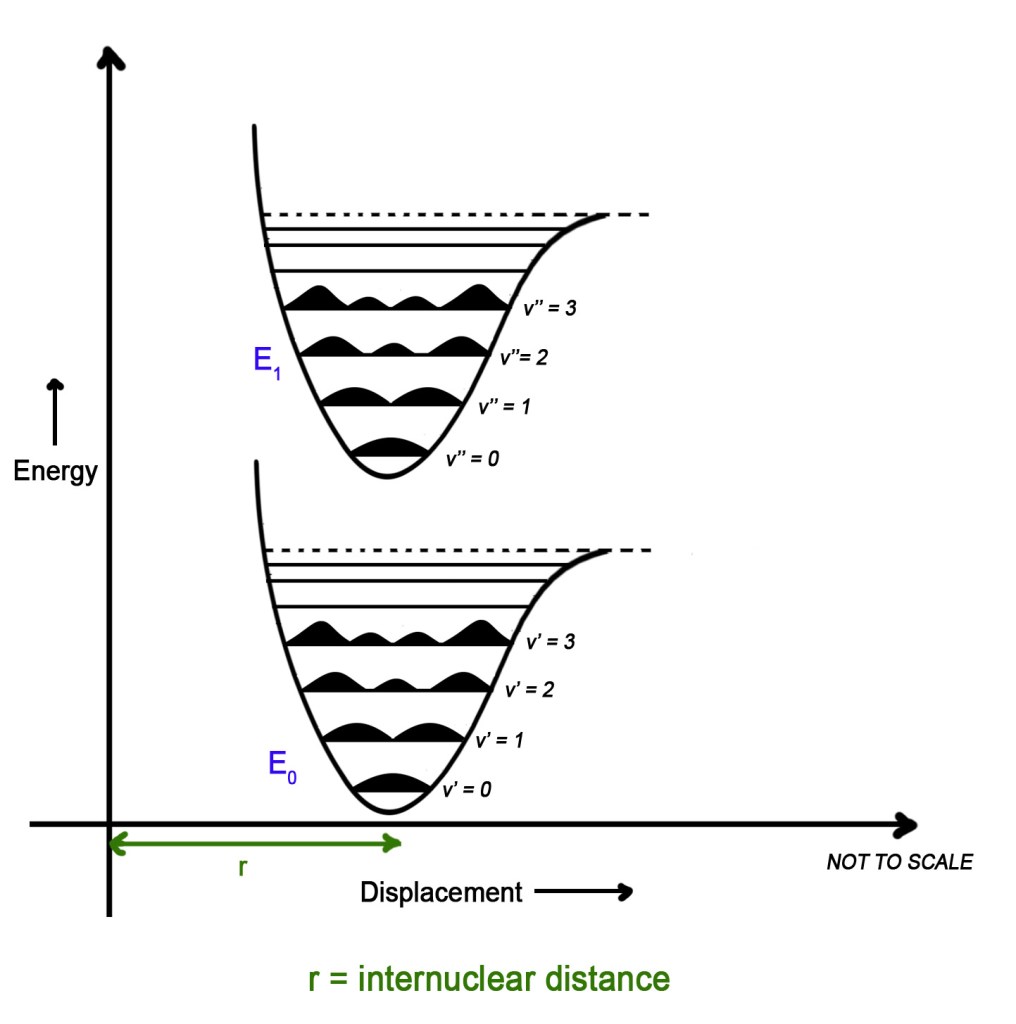
The internuclear distance/displacement for both E0 and E1 is the same (r).
In the next post, we will study how the transitions take place and find out why we don’t get equal probability peaks for transitions.Till then,
Be a perpetual student of life and keep learning…
Good day!