From this post onwards, we begin discussing the absorption of radiation by molecules in detail. In post 170, we discussed how absorption by atoms and molecules differs.
We studied that in atomic spectra, we encounter only electronic transitions. These transitions produce very sharp lines in the spectra.
As opposed to this, molecular spectra show many spectral lines- a band of frequencies. This is due to the fact that molecular spectra exhibit various electronic, vibrational, and rotational transitions. To know more about various types of motions in molecules read post 170.

ABSORPTION BY MOLECULES.
We learned in post 170 that, for a diatomic molecule, the total energy of the system is the sum of the translational, rotational, vibrational, and electronic energies of that molecule.
Etotal = Etrans +Erot+Evib+Eelec
Among these four, the rotational, vibrational, and electronic energy levels are quantized i.e they have certain discrete values. For all practical purposes, we DO NOT include translational energy in our discussions. The rotational and vibrational energy levels in a molecule can be shown as follows-
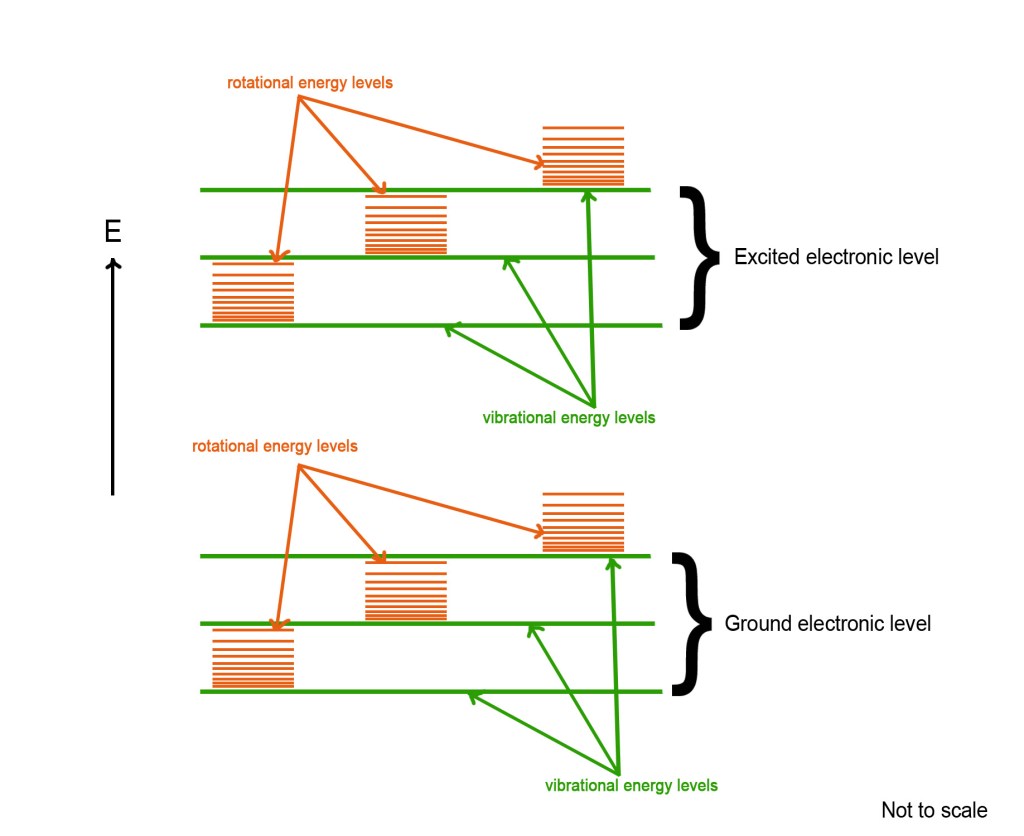
The above energy level diagram shows two electronic energy levels, namely – the ground state and the excited state. Each electronic level has several vibrational energy levels associated with it (shown in green color). Similarly, there are many rotational levels in each vibrational energy level (shown in orange color). These rotational levels are very closely spaced as the energy gap between them is very small. This explains the fine structure of the rotational energy bands.
It is clear from the above figure that the energy required by an electron, to transition in rotational levels is very less (as the rotational levels are closely spaced). The difference in energy between two consecutive rotational energy levels(ΔEJ) is roughly around 1cm-1. This energy corresponds to the energy of radiation in the microwave region. Thus, molecules absorb in the microwave region and electrons undergo rotational transitions.

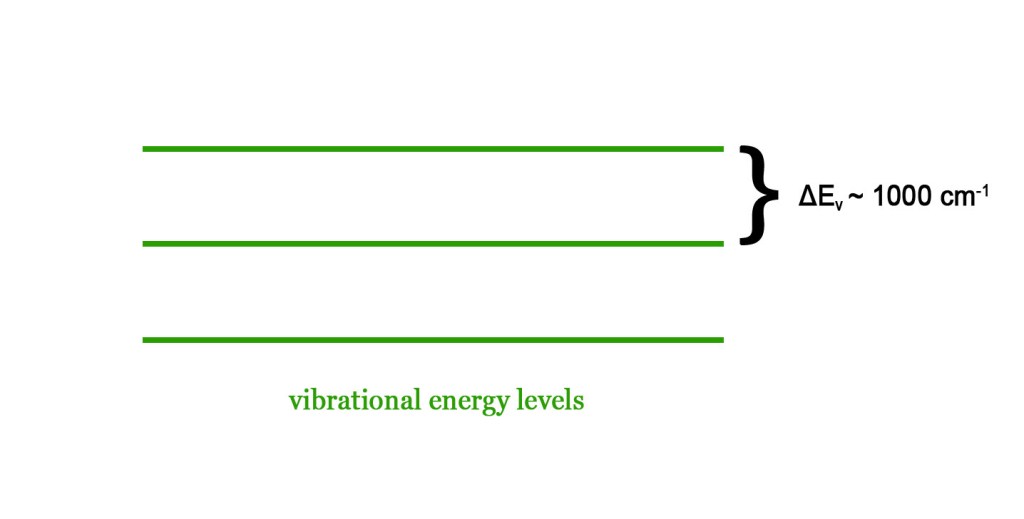
The energy gap between two consecutive vibrational energy levels is around 1000 cm-1. This is comparable to the energy in the IR region. Thus, IR radiation can bring about transitions in the vibrational energy levels. However, the energy in the IR region simultaneously also brings about transitions in the rotational energy levels. Thus, spectra in this range are referred to as vibrational-rotational spectra.
The electronic energy levels are much farther apart from each other. The energy difference between two consecutive electronic energy levels is approximately in the range of 10,000 to 100,000 cm-1. It takes radiation in the UV-visible range to excite an electron from the ground electronic state to the next electronic energy level. However, when we shine UV/visible radiation on a molecule, we see a variety of vibrational and rotational transitions too! Thus, we get a band of many spectral lines in molecular spectra, as shown in the figure at the top.

Thus, we can conclude –
Electronic level transitions – UV/visible region (~10,000 – 100,000 cm-1)
Vibrational level transitions – IR region (~ 1000 cm-1)
Rotational level transitions – Microwave region ( ~ 1cm-1)
The above data clearly shows that the energy associated with rotational energy levels is negligible when compared with the energies of vibrational and electronic energies. Thus, for all practical purposes, we neglect the rotational energy levels, while drawing an energy level diagram for molecules. Henceforth, we will only consider the vibrational and electronic transitions in our discussions.
In the next post, we will start discussing these electronic and vibrational transitions in detail. Till then,
Be a perpetual student of life and keep learning…
Good Day!