From this post onwards, we are going to study the most fundamental part of photochemistry – the absorption of radiation by atoms and molecules. What exactly happens when radiation is absorbed? How quantum mechanics applies to this absorption? What are the various photochemical process we encounter and how do they occur? We will answer all these questions and much more!
In post 155, we have already learned that radiation is absorbed only if its energy matches the difference in energy of the electrons in two energy levels. In the following figure, n=1 and n=2 are two electronic energy levels in an atom, with energy E1 and E2 respectively.

When radiation of different energy (a/b/c/d) is shone upon the atom, only a specific wavelength of radiation is absorbed (Note that as the energy of radiations is different, their wavelengths are different too. To know more about wavelengths read post 30).
The radiation with energy ‘a’ is absorbed by the adjacent atom because the difference in energy of two electronic energy levels, n=1, and n=2 exactly equals ‘a’ too (E2 – E1 = a). The other radiations are NOT absorbed as their energies are not equivalent.
Energy is absorbed both by atoms and molecules. We will study both these types of absorptions separately as the energy levels in atoms and in molecules are different. Why are energy levels different in atoms and molecules?
Atoms are solitary. We only deal with electronic levels when we consider atoms. However, in molecules, we have many kinds of motions with different energies. Thus, we have different energy levels. Thus, an energy level diagram for molecules looks more complicated than that of atoms.
Molecules are in a state of constant motion. The different kinds of motions in a molecule are as follows –
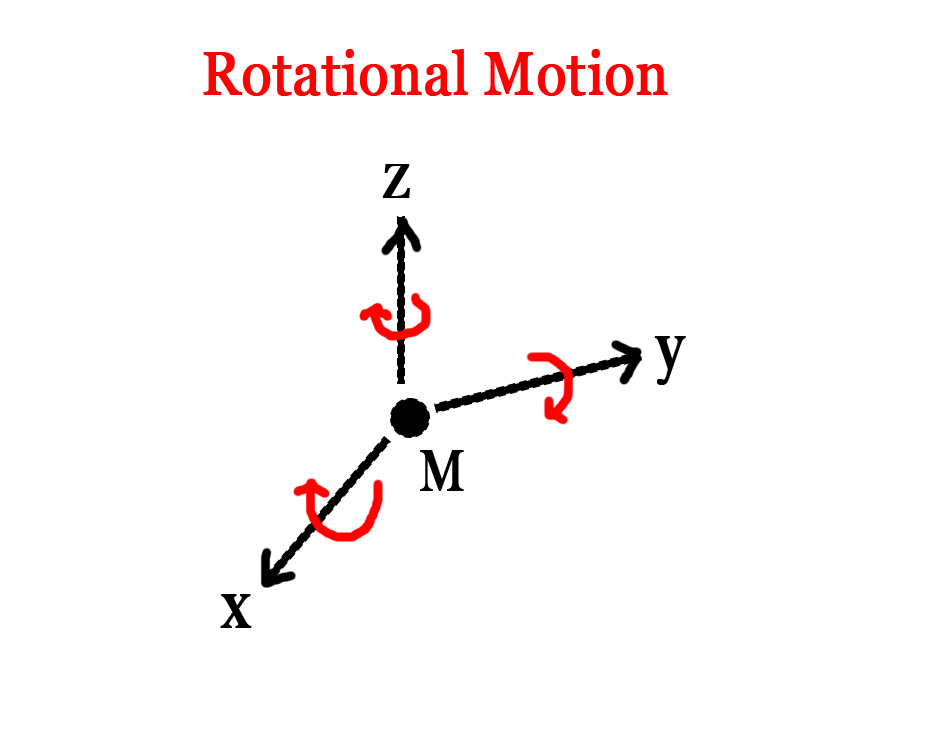
1)Translational energy(Etrans) – Molecules exist in a 3-dimensional space.The position of the molecule can be specified using the x-, y- and z- coordinates. However, they can displace themselves along these axes. This motion of molecules in a straight line, along the x-,y- or z-axis, is called translational motion. Translational motion results in a change of the x-,y-, and/or z-axis coordinates of the molecule. The energy associated with this kind of motion is referred to as translational energy. Thus, translational energy is a summation of the energy of a molecule along the x-,y- and z-axis.
Etrans = Ex + Ey +Ez
The translational motion of the molecule(M) along the three axes are shown as red arrows in the figure below –

Although this energy is quantized in space, its quantum effects are so small that they are not observable. Thus, for all practical purposes, it is considered that the translational energy of molecules is NOT quantized. We do not include this energy during calculations as the values of this energy are negligible when compared to values of rotational, vibrational, and electronic energies.
2)Rotational energy (Erot) – Molecules rotate around their internuclear axis, along x- , y- and/or z-axis. They behave as rigid rotors i.e their bond length remains fixed. The energy associated with this rotation is called rotational energy.
A non-linear molecule rotates along the x-, y- and z-axis as shown in the figure above. A linear molecule will rotate only around 2 axes, as the third dimension is missing.
3)Vibrational energy(E vib) – Molecules oscillate/vibrate about their internuclear axis. Molecules are harmonic oscillators. They can be thought of as springs, which vibrate along the internuclear axis.
What is a harmonic oscillator?
A harmonic oscillator can be thought of as a system vibrating with an equilibrium position and experiencing a restorative force(F) proportional to the displacement(x). Have a look at the spring below. The spring goes down and then a restorative force brings it back. The more the spring is stretched, the greater will be the displacement and thus the restorative force will be more too. This is an example of a harmonic oscillator. Mathematically, the expression for a harmonic oscillator can be shown as below- (the constant of proportionality(k) has a negative value as the system is brought back to its equilibrium position).
F ∝ x
∴ F = -k x
Similarly, consider a diatomic molecule(A-A). When the molecule is stretched, along the internuclear axis, two atoms will move farther apart from the equilibrium position and then come back to their original position due to the restorative force. Thus, it vibrates as shown in the figure below-

The energy of vibrating molecules is called vibrational energy(Evib).
4)Electronic energy (Eelec) – Atoms have orbitals, with specific energy, in them. We have already studied the Bohr model of an atom, which states that the electronic energy levels are quantized.
An electron in an orbital has a specific energy, equivalent to the energy of that orbital.
e.g.- the energy of an electron in the first energy level of a hydrogen atom is equal to -13.6 eV. Electrons absorb energy in the form of heat or light and get excited to higher energy levels. However, they fall back to lower energy states by giving away energy in the form of light/heat.
In the next post, we will continue discussing this concept. Till then,
Be a perpetual student of life and keep learning….
Good day!
References-

So nice. What about reaction of radiation in human body. As in Tata hospital, we have radiation dept..
LikeLiked by 1 person
studying radiation effects on human body is beyond the scope of chemistry syllabus here. That is a medical faculty discussion. I will however try to give a brief overview in upcoming posts 🙂 Thank you for your interest:)
LikeLiked by 1 person