We studied in the earlier posts that the Stark-Einstein law holds true only for the primary process of a photochemical reaction. However, we never studied what exactly a primary process means. Thus this post is dedicated to discussing the primary and secondary processes of photochemical reactions.
A primary process of a photochemical reaction refers to what happens immediately after absorption of light. This can be thought of as the first step of the photochemical reaction.
The subsequent chemical changes are termed secondary processes.
Let us try to understand this with an example – the decomposition of hydrogen bromide(HBr).

As seen in the above example, when an HBr molecule absorbs one photon of light (hν), it first decomposes to give hydrogen and bromine radicals. The free radicals are formed due to homolytic cleavage of the H-Br bond.
Homo = same lysis = breaking
If the electrons are evenly divided among the products, when the bond is broken, the cleavage is called ‘homolytic cleavage’.As seen in the above example, after absorbing a photon of light, the HBr bond breaks in a homolytic fashion. One electron goes with hydrogen to form hydrogen radical and another with bromine to form bromine radical. This is the first step or primary process of the reaction.
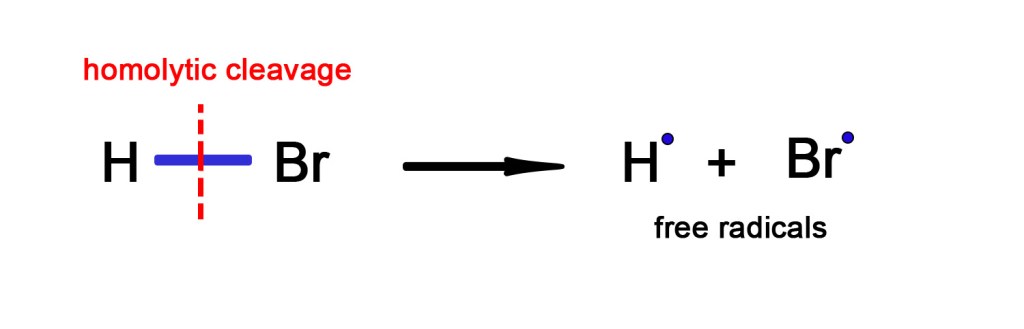
The subsequent steps are the secondary processes, where a hydrogen radical reacts with another HBr molecule to produce hydrogen gas. Also, the bromine free radicals react with each other to form bromine gas. Thus, all the free radicals produced are used up to form stable products (Free radicals are highly unstable and thus reactive).
The overall reaction clearly shows us that 2 molecules of HBr are decomposed by absorption of a single photon of light. Thus, the quantum yield for the overall reaction is 2.
Φ = # of molecules decomposed / # of quanta absorbed = 2/1 = 2. Thus, Φ >1.
However, for the primary process alone, the quantum yield is one. Thus, Stark-Einstein’s law is obeyed ONLY FOR THE PRIMARY PROCESS.

The primary photochemical process can be of two types –
1)Photophysical process
2)Photochemical process
Photophysical reactions –
These include radioactive transitions, where the excited molecule emits light in the form of fluorescence or phosphorescence and returns back to the ground state.No chemical reaction occurs in a photophysical process. We will study more about these in the next post.
Photochemical process –
In these reactions, the molecule after getting excited by the absorption of light undergoes a chemical change to give chemically different products. The excited molecule can isomerize, rearrange itself, dissociate or react with another molecule.
Let us study what each of these means –
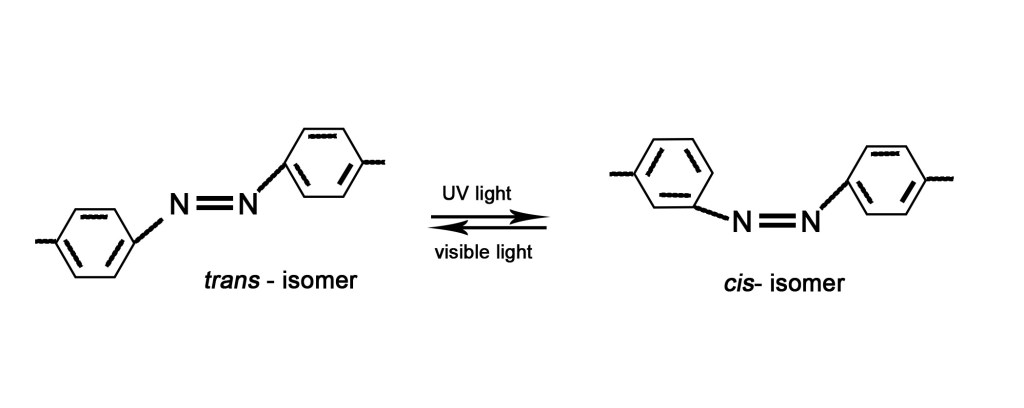
ISOMERIZATION – Isomers are compounds with the same composition but different structures. A compound can be converted to its isomer by absorption of light. As seen below, a trans-isomer can be converted to a cis-isomer by absorption of radiation. (A trans-isomer has the two benzene rings on the opposite sides of the double bond, whereas the cis-isomer has them on the same side). The cis- and trans- isomers have the same composition but their structures are different and so they are chemically different compounds.
REARRANGEMENT – This is also a kind of isomerization reaction, where the carbon skeleton of a compound changes to give a different structural isomer of the parent compound. As the carbon skeleton changes, we get a chemically different compound as the product. Thus, these types of reactions also come under photochemical processes.

As seen in the example above, a 5-membered ketone compound gets rearranged to a 6-membered aromatic phenol by absorption of radiation.
PHOTODISSOCIATION / PHOTOLYSIS –
Photo = light lysis/decomposition = breaking
When a bond is broken by absorption of radiation, it is referred to as photodissociation or photolysis.

As seen above, the HBr bond is broken to form two free radicals. These free radicals being very reactive will immediately react with other species in the reaction.
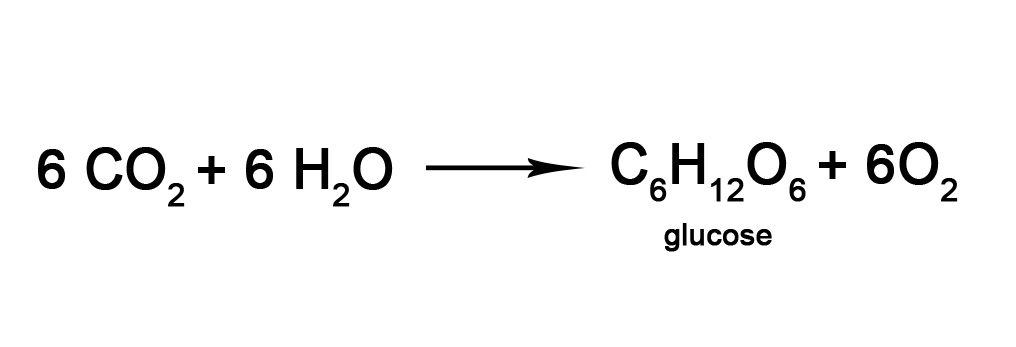
REACTION WITH ANOTHER MOLECULE – Two molecules react with each other in presence of certain specific frequencies of light to form new molecules. The best example of this kind of reaction is photosynthesis, where a glucose molecule is formed by using carbon-di-oxide (CO2) and water (H2O) molecule as shown below-
In this post, we discussed the primary and secondary processes of a photochemical reaction. In the next post, we will proceed to discuss the absorption of light and its consequences in greater detail. Till then,
Be a perpetual student of life and keep learning….
Good day!
Great Topic you have posted. It’s so beneficial for Chemistry students including me also. Thanks a lot to complete team who written this topic.
LikeLike
I am so glad you found it helpful 🙂 Thank you for your comment.
LikeLike