In the last post, we started talking about quantum yield. This quantity tells us how many molecules react by absorbing how many quanta of light.
As seen in the earlier post, quantum yield is defined as “the number of molecules of the substance undergoing the photochemical change per quantum of radiation absorbed.”
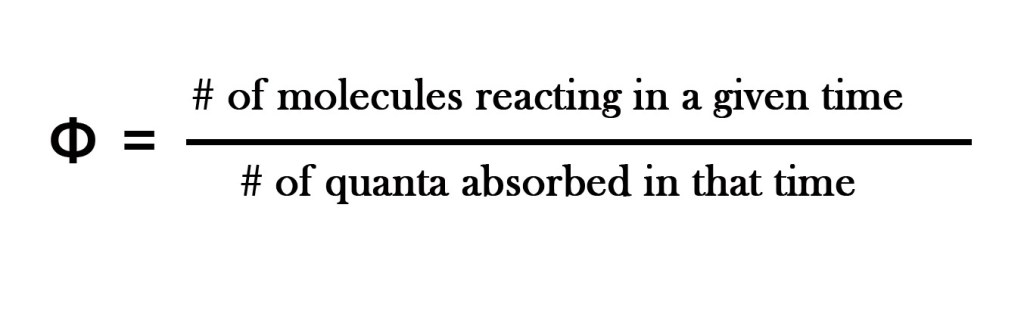
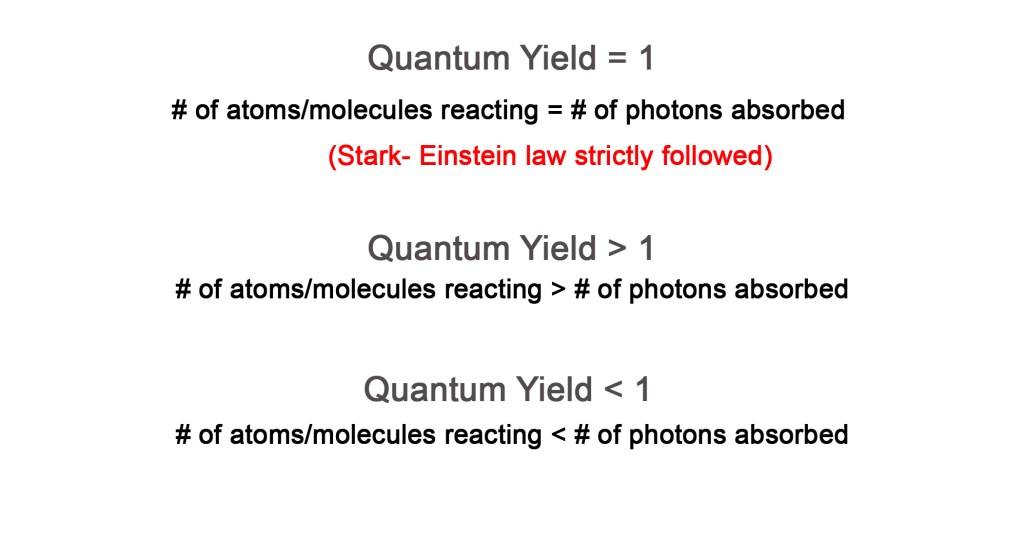
Thus, the quantum yield tells us the number of molecules undergoing a photochemical change per absorbed photon or the number of molecules decomposed or formed per absorbed photon. We also learned that for a primary process, that obeys Stark-Einstein’s law, QUANTUM YIELD IS ONE. This means that one molecule undergoes the desired photochemical change by absorbing one photon.
However, there are photochemical reactions that show exceptions to this behavior i.e they do not obey Stark- Einstein’s law. For such reactions, the quantum yield is NOT one. Let us study these exceptions –
EXCEPTION 1 – Quantum Yield is more than 1 (Φ>1).
In some photochemical reactions, involving intense lasers and for some radiation-induced chain reactions, a single photon might trigger a series of chain reactions. Thus, absorption of a single photon leads to the decomposition of two or more molecules. In this case, Φ>1
Let us assume that 2 molecules decompose by absorbing one photon, then the quantum yield will be –
Φ = # of molecules reacting / # of quanta absorbed = 2/1 = 2. Thus, Φ >1.
Thus, when the quantum yield is more than one, the number of molecules reacting is much larger than the number of photons absorbed.
The reasons for high quantum yield are –
1)secondary reactions occur after the primary one as shown below –

As seen above, when a molecule AB absorbs radiation (hν), it breaks down to form free radicals of A and B. Later, the free radical of A attacks another AB molecule to produce A2 and one more free radical of B. This is a secondary reaction, which causes the quantum yield to be more than 1.
2)a chain reaction is initiated which produces many products per photon –
When there is more than one reactant, we often see chain reactions, which leads to higher quantum yields.
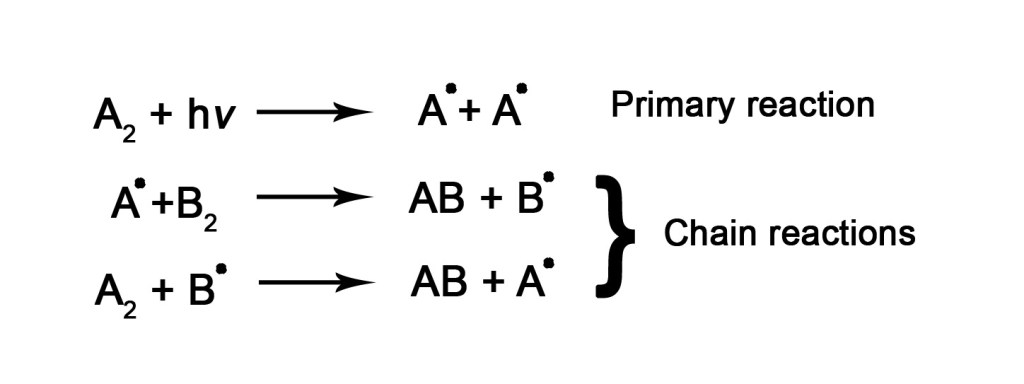
In the above set of reactions, there are two reactants namely A2 and B2.In the primary step, A2 absorbs radiation and decomposes homolytically (to read more about homolytic cleavage, click here), to give two free radicals(A•). These free radicals are highly unstable and they immediately react with the other reactant B2 to form product AB. However a free radical, B•, also gets formed, which then attacks another A2 molecule, to form AB product and A•. This A• reacts with B2 again and this chain of reactions continues to proceed. Thus, by absorption of just a photon(hν), a series of reactions occur. This results in a quantum yield greater than 1.
EXCEPTION 2 – Quantum Yield is less than 1 (Φ<1).
This exception is opposite to the earlier one. Here the quantum yield is less than one because the number of molecules reacting is much less than the number of photons absorbed.
The reason for low quantum yields are –
1)the activated molecule gets deactivated before forming products as shown below–
M* → M + hν where,
M* – Excited molecule
M – Deactivated molecule
hν – emitted light
2)excited molecules collide with non-excited ones and transfer their energy to them.
M*+ A→ M + A* where,
M* – Excited molecule
A – Another molecule
M – Deactivated molecule
A* – Another molecule in the excited state.
3)the decomposed fragments may recombine to form the reactant again. Thus, no new products are formed.
A → a + b
a + b → A where,
A – Reactant molecule
a , b – Decomposed fragments.
We have already stated that the Stark Einstein law holds true only for the primary process of a photochemical reaction. But what is a primary process? We shall learn more about the primary and secondary photochemical processes, in the next post. Till then,
Be a perpetual student of life and keep learning…..
Good day!