In the previous post, we learned why energy level diagrams in atoms and molecules are different. We can summarize the post by concluding that, in atoms, we need to look Only at the electronic levels (as atoms are solitary). However, for molecules, we need to take into account the rotational, vibrational, and electronic levels.
ATOMS ⇒ Only electronic levels ⇒ comparatively simple energy level diagrams.
MOLECULES ⇒ Electronic + vibrational + rotational levels ⇒ Complex diagrams
Let us now proceed to look at these energy level diagrams and understand the absorption process for atoms and molecules.
ABSORPTION BY ATOMS
In atoms, the transitions of electrons happen among the electronic energy levels. These electronic transitions can be graphically shown by Grotrian/term diagrams.
Grotrian/Term diagrams
These diagrams are named after the German astrophysicist Walter Grotrian.
The electrons in atoms occupy the atomic orbitals (AO).A single atom can have one (hydrogen atom) or many electrons. Then again, each electron has 4 quantum numbers! These electrons sit in various energy levels according to how much energy they possess. Without a clear diagram, it would be very difficult to visualize their positions and transitions in atoms.Grotrian/term diagrams help us in this regard.
These diagrams give us a detailed visual of the complex level structure of multi-electron atoms and show us the allowed transitions between atomic energy levels.
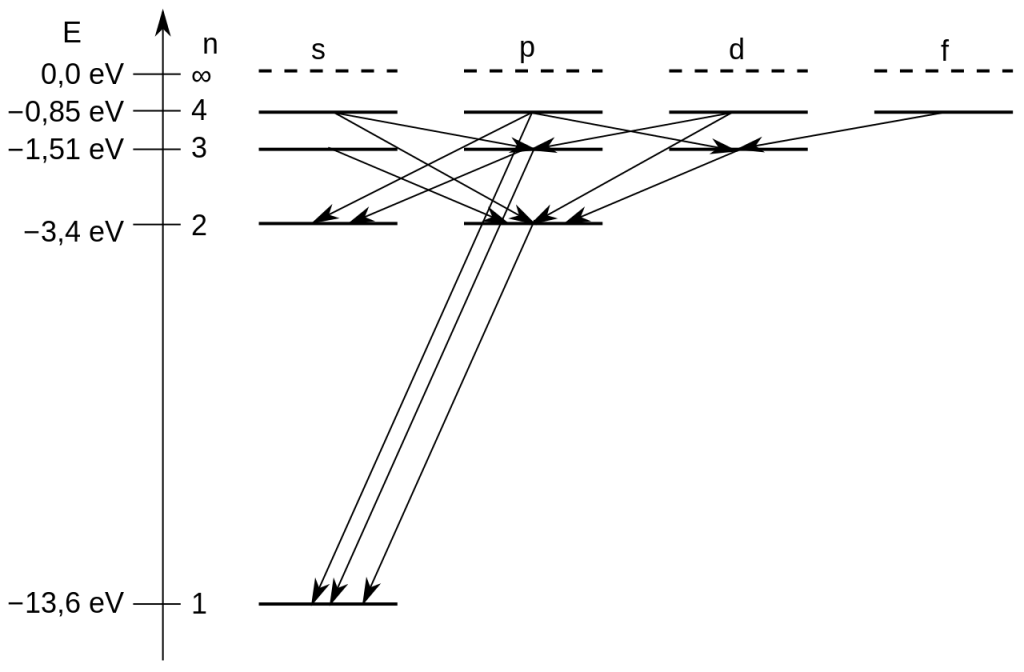
Let us study a Grotrian diagram for hydrogen atom –
The above Grotrian diagram shows all the allowed transitions for an H-atom. We already know that, when an electron absorbs radiation, it gets excited from a lower energy level (ground state) to a higher energy level (excited state). These transitions are induced by photons of specific wavelengths. Reverse transitions also occur – the excited electron in the higher energy state tends to fall back to the lower energy state by releasing the excess energy.
From a quantum mechanical point of view, these transitions follow certain selection rules.
Selection rules for electronic transitions –
Rule 1 – Transitions that follow the Δ𝓵 = ±1 rule. Δ𝓵 =0 is FORBIDDEN.
The ‘𝓵’ in this rule refers to the azimuthal/orbital quantum number. To know more about quantum numbers read post 27.
+𝓵 ⇒ electron goes from a lower energy state to a higher energy state.
-𝓵 ⇒ electron goes from a higher energy state to a lower energy state.
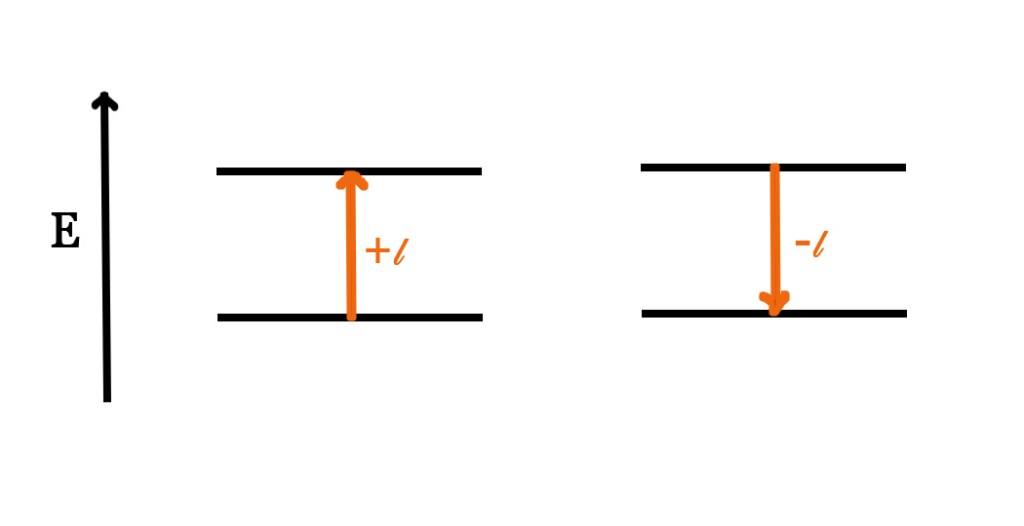
Rule 2 – Change in magnetic quantum number Δm = 0, ±1.
To illustrate these rules, we again consider the one-electron H-atom. Consider a transition from the diagram above (highlighted in orange color)-

This is a reverse transition, where the electron is moving down from the 2p level to the 1s electronic level (ground state).
n ⇒ principal quantum number
𝓵 ⇒ azimuthal/orbital quantum number
m ⇒ magnetic quantum number.
For 1s electronic level, n=1, 𝓵=0, m=0
For 2p electronic level, n=2,𝓵=1, m=0(for y-axis) ,m=+1(for z-axis),m=-1(for x-axis)
Suppose the electron under consideration is in the 2pz axis. Thus,m=+1.
Then, Δ𝓵 = 1–0 =1.
Also, Δm =0–0=0. Thus, this is an ALLOWED TRANSITION. (Check the colors to understand how the values of quantum numbers are substituted).
Can an electron go from a 2px to a 2pz orbital?
For both 2px and 2pz, the orbital quantum number,𝓵=1.
∴Δ𝓵 = 1-1 = 0. Thus, this is a FORBIDDEN TRANSITION.
As mentioned in several posts before, in quantum mechanics we are never 100% sure of the outcome. We only talk about probabilities. Thus, in quantum mechanical terms, the allowed transitions are the ones that are more likely to occur. Similarly, the occurrence of the forbidden transitions is a low probability event.
ALLOWED TRANSITIONS ⇒ High probability transitions (more likely to occur)
FORBIDDEN TRANSITIONS ⇒ Low probability transitions (less likely to occur)
Check out this video for more information on selection rules.
We studied the simplest Grotrian diagram for a one-electron H- atom. However, for multi-electron atoms, these diagrams get very complex.
Now that we have a thorough understanding of energy level diagrams for atoms, we shall proceed to discuss the energy level diagrams for molecules in the next post.
Till then,
Be a perpetual student of life and keep learning…..
Good Day!