In the previous post, we concluded the discussion on the Franck-Codon principle. This principle states that the molecule’s geometry remains unchanged during an electronic transition. At this point, we proceed to study the electronic transitions. Before exploring these transitions, a clear understanding of electron spin and the associated singlet and triplet states is necessary.. Let us begin our discourse on this concept.
THE ELECTRON SPIN
Properties of any particle/object are of two types-
- Intrinsic properties – Properties that are inherently there in the particle. These properties exist because of what the particle is. e.g- Charge. An electron has a negative charge – it just has a negative charge in it.
- Extrinsic properties – Properties that depend on what the particle/body is doing at that given moment of the time.e.g.-Linear momentum, which is the momentum of an object moving in a straight line i.e linearly. This property exists because the object is moving at that time.
Electrons have 3 inherent or intrinsic properties-
- Mass (9.1× 10 -31 kg)
- Charge (1.62 × 10-19Coulombs)
- Spin
In post #27, we studied the spin quantum number. Every electron has a spin and this is an inherent property. The spin of an electron can actually be thought of as its intrinsic spin angular momentum.
However, does the electron really spin?
If we assume that electrons are solid spheres (we know that electrons can be modeled as both particles and waves– wave-particle duality), the diameter of an electron is approximately 10-18m. If an electron actually spun around its axis it would have to do it superluminally (more than the velocity of light) to have the right magnetic moment and angular momentum. According to calculations, if the electron was spinning, it had to spin with a velocity- a million times that of the velocity of light (c = 3× 10 8 m/s). However, we know that nothing can go faster than the velocity of light, as time and space do not exist beyond this point. Thus, we can conclude that AN ELECTRON DOES NOT ACTUALLY SPIN.
So then why do we say that this spin exists? It is because the electrons are affected by magnets. Any moving charge (electrons in this case), with angular momentum, is going to have a magnetic field. This magnetic field will be affected by another magnetic field; electrons are deflected by an external magnetic field. Thus, they behave as tiny magnets. An electron has a magnetic moment associated with its intrinsic spin. Therefore, we have to believe a ‘spin’ exists in electrons!
Electron spin has two orientations- clockwise and anti-clockwise. Spin is a quantized quantity meaning an electron can have only two discrete spins. This means that an electron behaves as if it is either spinning clockwise or in the anti-clockwise direction as shown below-
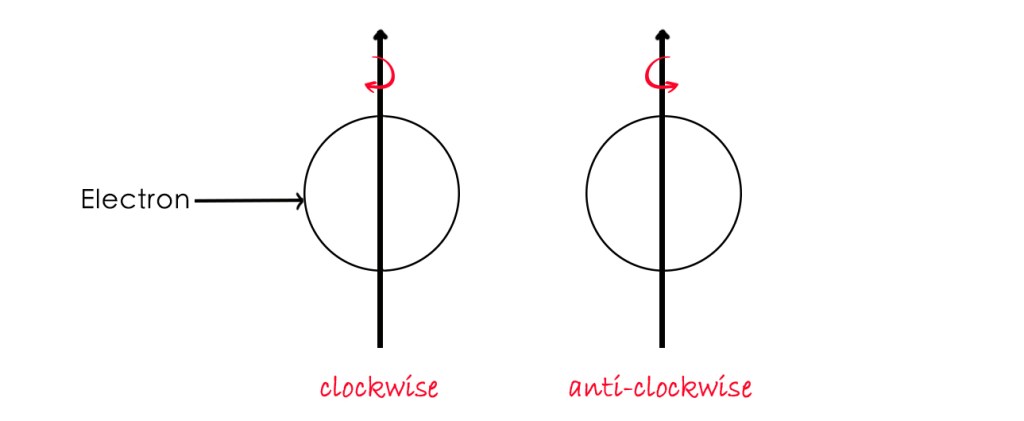
The spin of an electron has a significant role to play in chemistry and quantum mechanics, as it helps explain many phenomena accurately. We shall talk more about the electron spin in the next post.
In photochemistry, the spin of electrons decides the singlet or triplet state of electrons, which is a very important concept. In the next post, we shall delve into this topic in detail.
Till then,
Be a perpetual student of life and keep learning…
Good day!
.
interesting notes
LikeLike
nice
LikeLike
nice notes
LikeLike
I am glad you found it helpful!
LikeLiked by 1 person
are you going to write about coordination compounds
LikeLike
are you going to write about coordination compounds
LikeLiked by 1 person
please tell
LikeLike
Hi.. I will eventually 🙂
LikeLiked by 1 person