This is the concluding post on the periodic properties of elements. The property we will talk about in this post is – the melting point(M.P).
The Melting Point (M.P)
The temperature at which a solid converts into a liquid state is called its melting point.
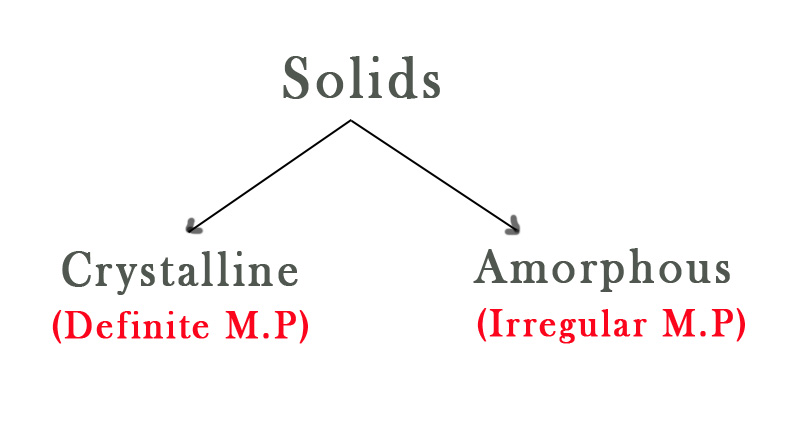
Solids are of two types- Crystalline and amorphous.
Crystalline solids have a definite structure as the components are arranged in a regular pattern in a crystal lattice. A lattice is a three-dimensional arrangement of atoms in a solid. It is the framework of the components of the solid. The components of that solid(atoms/ions/group of atoms) are represented as points in the lattice. There are 7 lattice patterns that we will discuss when we study solid-state chemistry. All the solids are arranged in one of these 7 ways. As the crystal has a definite arrangement, the components are at the same distance from the same type of neighboring components. Thus, the intermolecular forces holding them together in the crystal is uniform. This is the reason why crystalline solids have definite melting points.
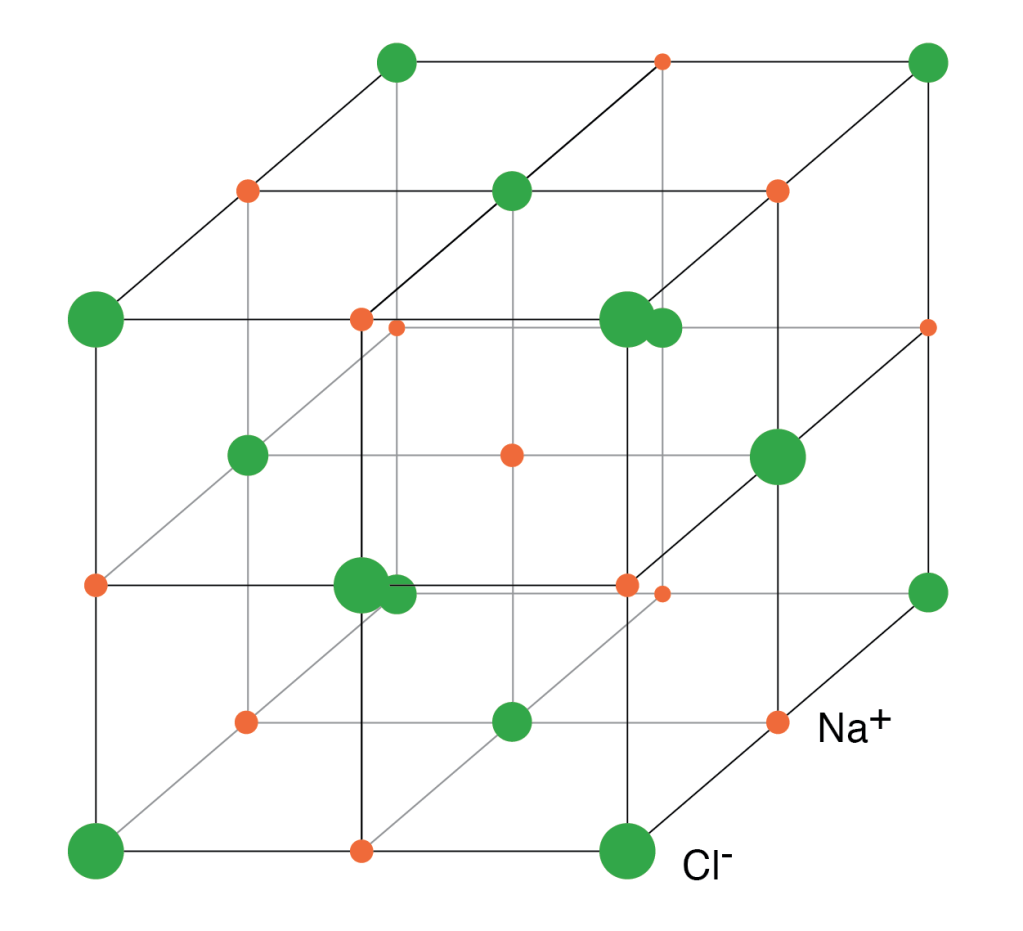
The adjacent figure shows us the lattice structure of common salt or NaCl molecule. The orange points represent sodium ions(Na+), and the green ones represent chloride ions(Cl–). All the ions are arranged in a specific 3D pattern.
Each ion has 6 nearest neighbors of a different ion and 12 of the same kind as its second nearest neighbor. This means that if you take any sodium ion, it will be surrounded by 6 chloride ions. These are its nearest neighbors. It will have 12 sodium atoms as its second nearest neighbor.
If you take a crystalline solid and heat it, the temperature of the solid begins to rise. The heat increases the kinetic energy of the atoms/ions in the solid. This causes them to vibrate faster in the crystal lattice. After a while, some of the components vibrate so fast that they break free of the lattice. At this point, the crystal structure starts to break apart. The bonds are broken, and the solid starts melting and entering a liquid state.
A thing to note here is that the stronger the bonds between the components of a solid, the harder it will be to break them. This means that more energy will be required to break strong bonds. Thus, solids with strong bonds have high melting points.
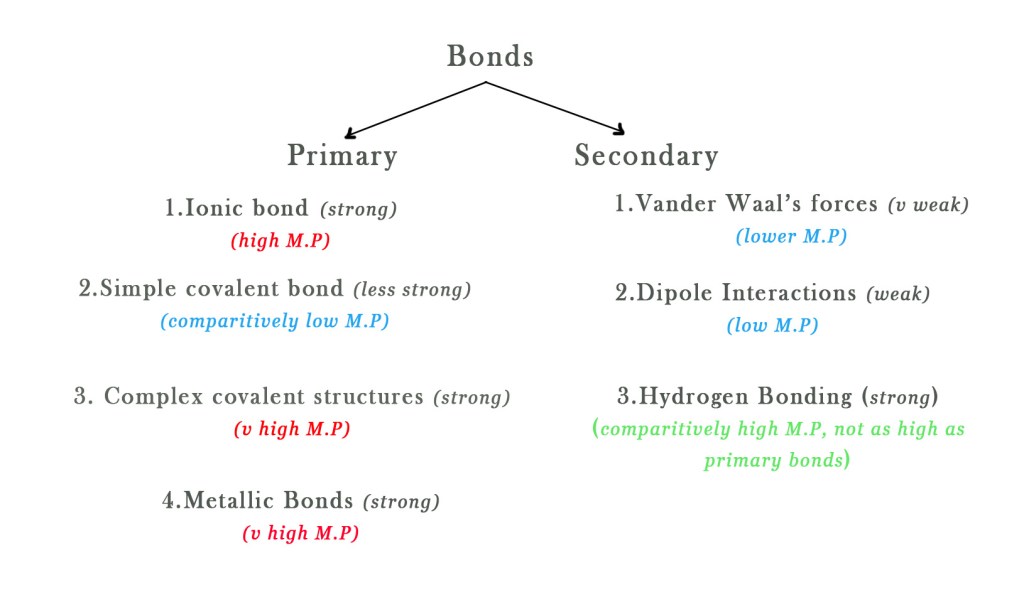
There are many types of bonds in solids – Primary bonds and secondary bonds. To know more about these bonds in detail read post #54.
In general, in primary bonding, solids with ionic and metallic bonding have high M.Ps. Molecules with simple covalent bonds have lower boiling points. Molecules with complex covalent bonds have higher M.P.
e.g.–
1) Ionic Solid – Sodium Chloride (NaCl) M.P = 801 °C (High)
2) Metallic solid – Iron (Fe) M.P = 1538 °C (very high)
3) Simple covalent solid– Benzoic acid ( C6H5COOH) M.P = 122 °C (comparatively low)
4) Complex covalent solids – Graphite M.P = 3600 °C (very High)
As far as the secondary bonding is concerned, the bond strength increases as follows-
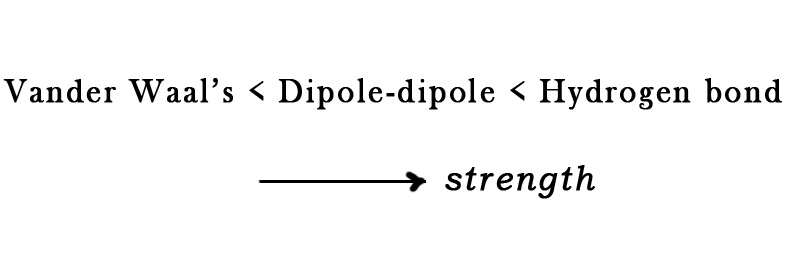
Thus, solids with a hydrogen bond will have higher M.P. than those with weaker secondary forces in them. However, secondary bonding is weaker than primary bonding. So, generally, the secondary bonds (except hydrogen bonds) have little effect on the melting point.

During melting, the temperature remains constant as all the heat absorbed is utilized to bring about the change in state. The temperature remains constant till all the solid has melted.
Amorphous solids are non-crystalline solids that lack long-range order. These solids are made by cooling down the molten state very quickly. The time crunch doesn’t allow the components to arrange themselves properly into a thermodynamically stable crystalline state. They can also be made by adding substances that prevent crystal formation. As their structure is not definite, they do not melt at a specific temperature. They melt over a range of temperatures.
e.g.- Window glass, cotton candy.
The trends in the M.P
There is no specific trend in M.P. of elements of the periodic table. However, as chemists, we must generally know the pattern. The following chart gives us an idea of the M.Ps of the elements in the periodic table.
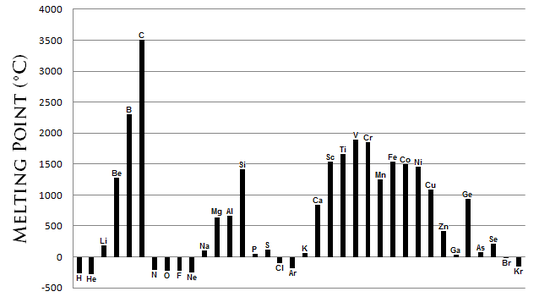
As seen in the above figure, the metals (e.g.- B, Be, Sc, Ti, Fe, V, Cr, Mn, Cu, etc ) have high melting points. This is because they have metallic bonds in them. The metallic bonds are pretty strong and more energy is required to break them.

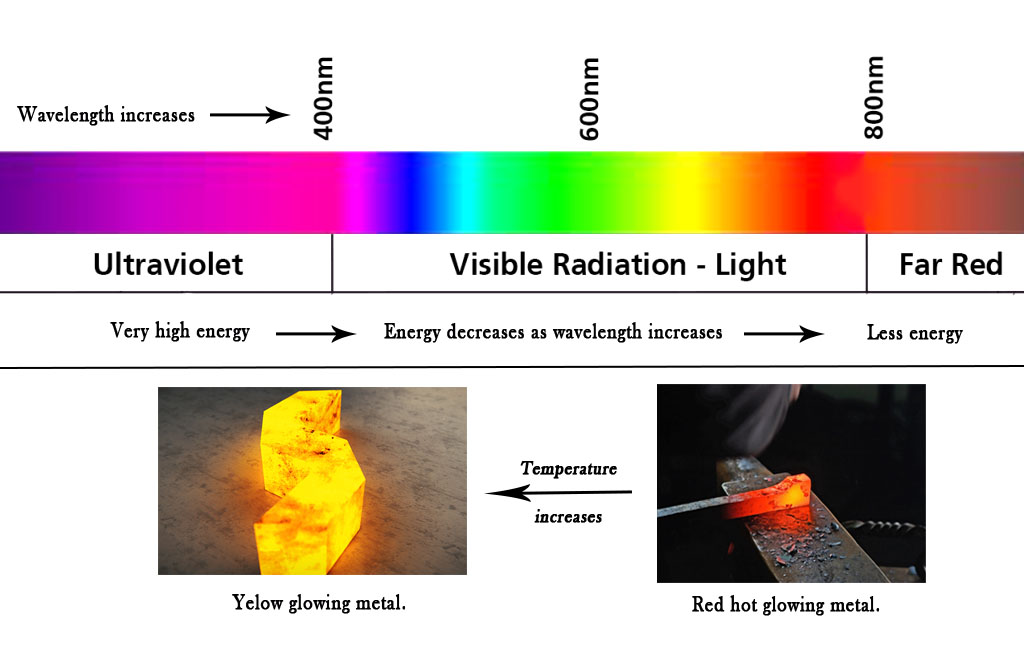
Iron(a metal) melts at 1538° C !! To have a sense of scale, think of how hot one feels when the summer temperatures rise to 45° C! Generally, all substances emit thermal radiation but the wavelength is far less than that visible to the human eye. However, when iron melts it does so by emitting radiation in the visible range. That is why we see the yellow color of the molten iron.
However, when the piece of metal is heated it starts to glow (black body radiation) due to thermal excitation of electrons. It first becomes red hot. This is because the red color has a higher wavelength (lower energy). Later it starts turning orange and then yellow. This phenomenon shows us that as the temperature rises, the wavelength of light emitted becomes less.
Temperature (T)∝ Energy(E) ∝ (1/Wavelength ,λ)
Temperature and energy are directly proportional to each and they both are inversely proportional to the wavelength of light emitted. This means the higher the temperature, the lesser the wavelength will be (As seen in the figure below, at lower temperatures the metal glows in red color. As the temperature increases, it starts glowing in yellow color). Substances that melt at a higher temperature than iron will emit in the UV range. However, our eyes cannot see those radiations.
Non-metals(H, S, Se, etc) generally have lower M.P. However, CARBON(non-metal) HAS THE HIGHEST M.P. of all elements! This is due to a property it possesses called ‘Catenation’, where C-C covalent bonds can be formed. The carbon structures have extensive bonds in them and so is high melting.
Second Row Elements Trend –
Carbon is a second-row element. The trend in these elements can be graphically represented as follows –
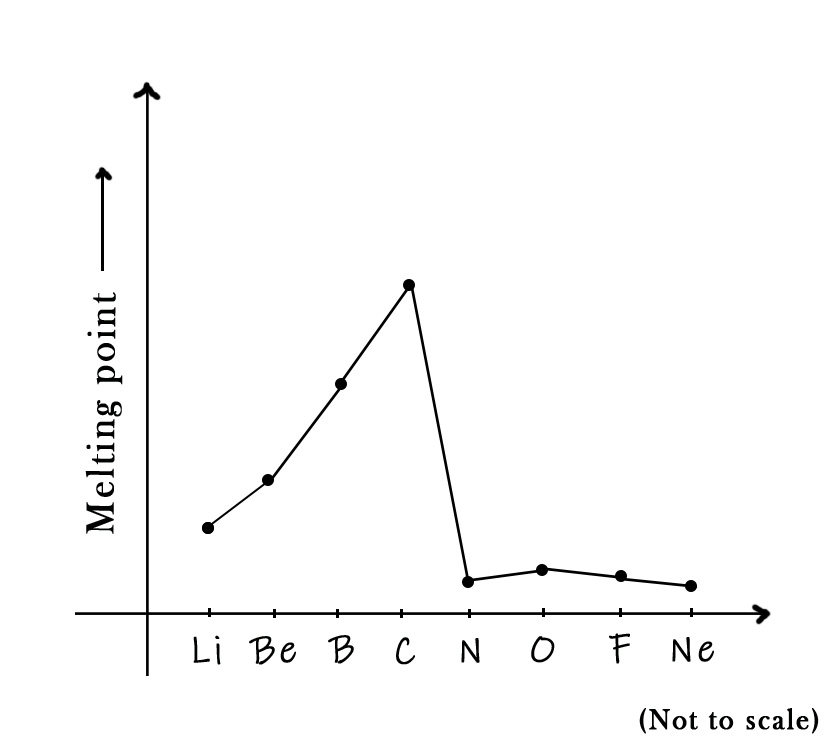
As seen in the figure above, the melting point temperature gradually increases from lithium to beryllium. Both of these elements are metals and so have expected melting points. Boron is a metalloid. It has small size and has a closed-pack structure. The boron atoms are tightly bound in the crystal lattice, and thus their melting point is higher. Carbon, as discussed earlier, is a non-metal with an exceptionally high melting point.
As we move from carbon to nitrogen, the melting point temperature drops drastically. This is because nitrogen has simple covalent bonds in the N2 molecule. Molecules with simple covalent bonds are generally low-melting solids. Oxygen has a slightly higher mass than nitrogen, thus the Vander Waals forces are a bit stronger. Thus, its melting point is slightly higher than that of nitrogen.
Third Row Elements Trend-
The third-row elements( Na, Mg, Al, Si, P, Cl, Ar) show melting point trends similar to the second-row elements, with some variations.
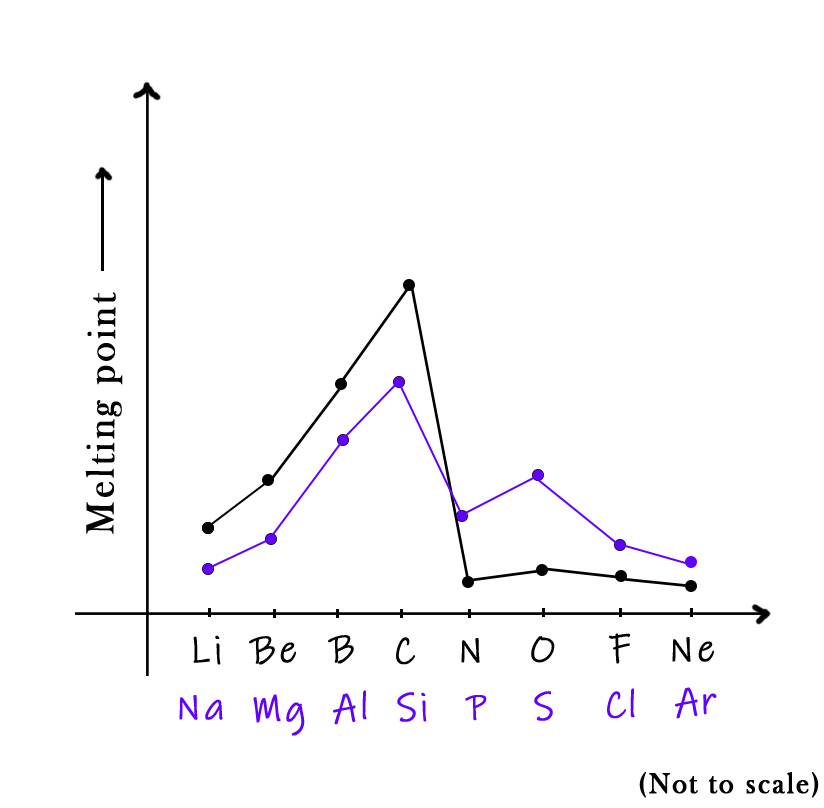
Sodium, magnesium, and aluminum are metals. Silicon is a metalloid. It forms complex structures like carbon too. This it’s a high-melting solid element. Phosphorous is a non-metal and so the melting point temperature drops here. However, as compared to nitrogen, its melting point is higher. This is because nitrogen forms an N2 molecule, whereas phosphorous forms a P4 one. So, the phosphorous molecule has 4 covalent bonds, which need more energy to break. Moving to sulphur we see a significant rise in the temperature. This is because sulfur forms an S8 molecule. All the eight bonds in this molecule are sigma bonds, which are hard to break. Thus, its melting point temperature shoots up. Chlorine and argon show an expected trend.
In general, M.P. depends upon a number of factors and so a proper trend is not seen for all elements.
In conclusion, we can confer that, the melting temperature is indicative of the energy that is required to break the bonds between the atoms in the solid. The more tightly packed the solid structure is, the more will be its melting point, as it will require more energy to break the strong bonds in it.
Thus, high bond dissociation energy is directly proportional to the melting point temperature of solids. We have already used this term – bond dissociation energy- in the previous post. Let us study in detail what this actually means.
With this post, we end our discussion on the different properties of elements and their periodic trends. In our next post on the periodic table let’s try to find out how accurate this table is in the real sense. Till then,
Be a perpetual student of life and keep learning…
Good Day!
References and further reading –
1.https://en.wikipedia.org/wiki/Bond-dissociation_energy
2.https://chem.libretexts.org/Core/Inorganic_Chemistry/Descriptive_Chemistry/Periodic_Trends_of_Elemental_Properties/Periodic_Trends
Image source –
1.https://chem.libretexts.org/Core/Inorganic_Chemistry/Descriptive_Chemistry/Periodic_Trends_of_Elemental_Properties/Periodic_Trends
2. Wikimedia Commons – Melting of Iron
3.https://www.craftinireland.com/events/details/international-forge-in-june-2011/
4.https://www.behance.net/gallery/15090527/Glowing-Metal
5.https://www.allaboutcircuits.com/uploads/articles/rigid-crystal-structure.png