P3.THE METALLIC BOND.
In this post, we shall discuss the last kind of primary bond – The metallic bond.

We see many objects in our everyday life which are made of pure metals like gold bars/coins, copper wires, etc. All these objects have atoms of the pure element held together by some force. What is this force? How do metal atoms bond with each other? The answer to this question is -the metallic bond. A metallic bond is a force that binds several metal atoms together in a metallic solid.

Metals showed some exceptional behavior and the traditional bonding theories at that time could not explain it. The discovery of metallic bonding started with certain observations. The observations indicated that the bond between two metal atoms is neither ionic nor covalent.
The observations made were as follows-
OBSERVATION # 1 – Values of Electrical conductivity.
Electrical conductivity values show how well a substance allows electricity to pass through it. The higher the electrical conductivity values better the substance is at letting electricity pass through it. This means that good conductors of electricity have greater electrical conductivity values.
Scientists were looking at the electrical conductivity values of elements. And they observed that metals have higher electrical conductivity values than other elements.
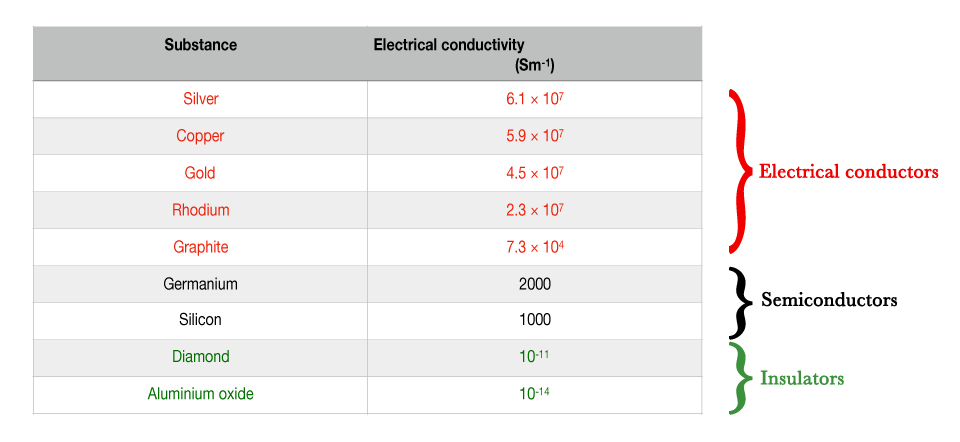
The electrical conductivity value is expressed in Siemens per meter(S/m).
As seen in the above table,
The value for silver (metal) =6.1 × 107 = 61000000 S/m
The value for Germanium (metalloid) =2000 S/m.
That was a huge difference!
As seen in the above table, the metals have very high values of electrical conductivity. It was experimentally confirmed that metals can conduct electricity very efficiently. The question was why do metals show this behavior?
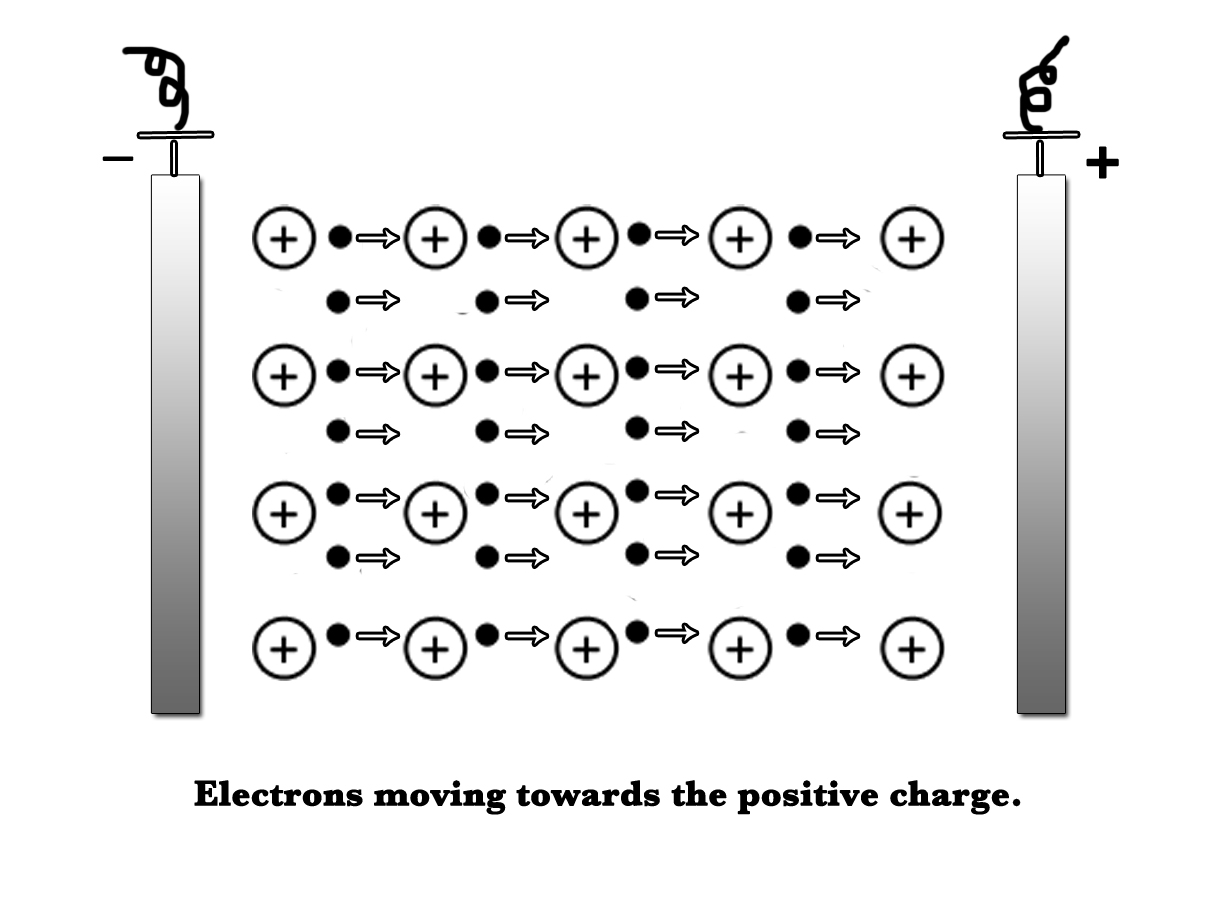
The conduction of electricity is related to the free electrons in any structure. It is the movement of the free electrons that produces electricity. Free electrons are the electrons that are not bound and can move around freely in the lattice. The ionic and covalent compounds have valence electrons that are tightly bound and so these compounds are not good at conducting electricity. Thus, it was concluded that there had to be a different kind of bond in metals, that could explain this observation.
Note – Graphite is the ONLY NON-METAL with high electrical conductivity. This is due to its structure.
OBSERVATION # 2 – Metals are malleable and ductile.
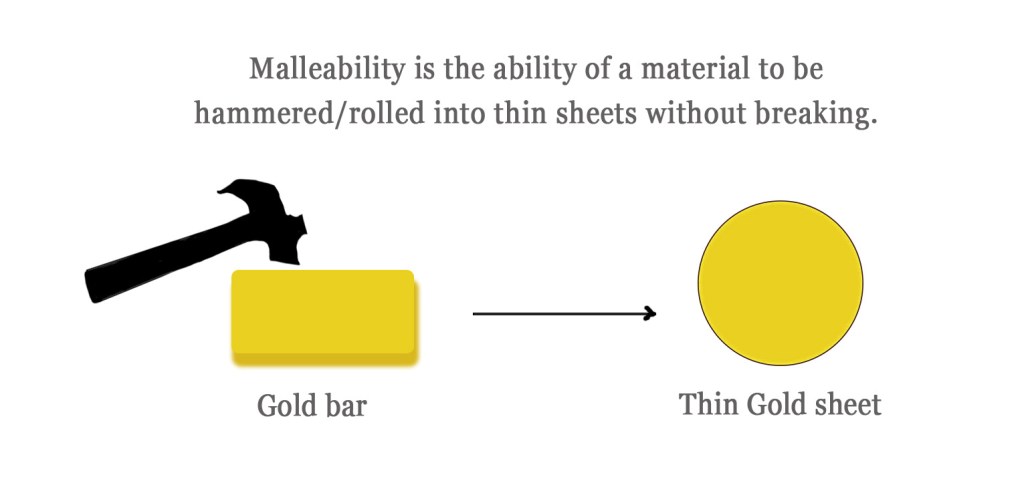
Malleability is a physical property of a material that describes its ability to deform under pressure, without breaking. A malleable material can easily be converted into thin sheets by hammering.
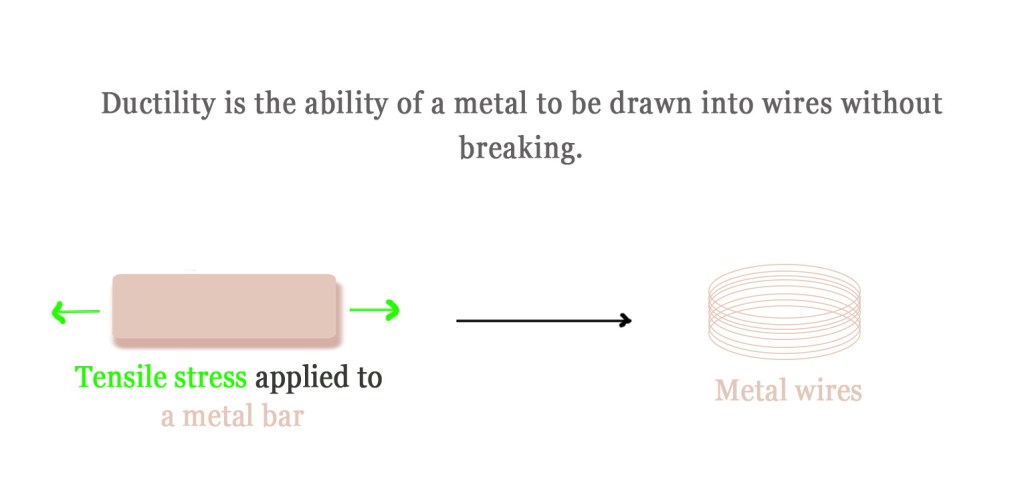
Ductility is another physical property that is related to the ability of materials to undergo plastic deformation without breaking. Plastic deformation is a process by which a material changes its shape permanently due to the tensile stress applied to it. Tensile stress means any force that pulls two ends of an object away from each other (like in tug-of-war). When such a force is applied to ductile materials, they may bend, twist, or get drawn into wires, but WILL NOT BREAK.
Metals are both malleable and ductile. They can be beaten into sheets and drawn into wires. For such a phenomenon to occur, the layers of the metals in the lattice must glide over each other easily. No bonding theory could explain how that would be possible.
These unique properties shown exclusively by metals forced the scientists to develop a befitting model for bonding in metals. In the 1900s, it was Paul Drude who came up with a theory for explaining bonding in metals.
The Electron Gas Model

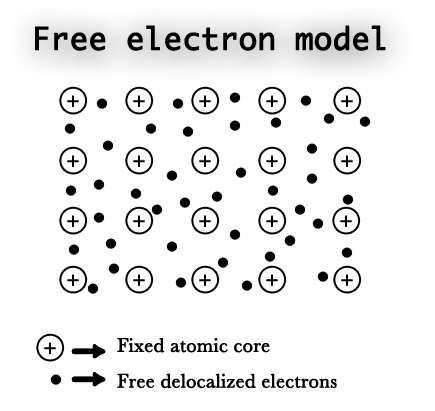
German physicist, Paul Drude, introduced the concept of metallic bonding. He proposed a model, which helped explain some properties of the metals. This model stated that the metal atoms consist of two parts –
1) The Atomic Core ⇒ includes the nucleus of the atom and all its inner shell electrons.
2) The Valence electrons ⇒ the outermost electrons of the atom.
According to this model, the inner electrons remain bound to the nucleus and the valence electrons are delocalized i.e. they are not on any single atom. This model considers the lattice as being made up of metal ions surrounded by a sea of delocalized electrons. The metal ions can vibrate in their respective position, however, their position remains fixed. The outermost delocalized electrons do not have a fixed position and are free to move throughout the lattice. These delocalized electrons are responsible for the exceptional behavior shown by the metals.
High Electrical conductivity values – The delocalized electrons in a metal lattice move in a random direction, but when a potential difference is applied across the metal, they start moving from a higher potential to a lower potential. This movement of the electrons produces an electric current. Thus, metals are good conductors of electricity.
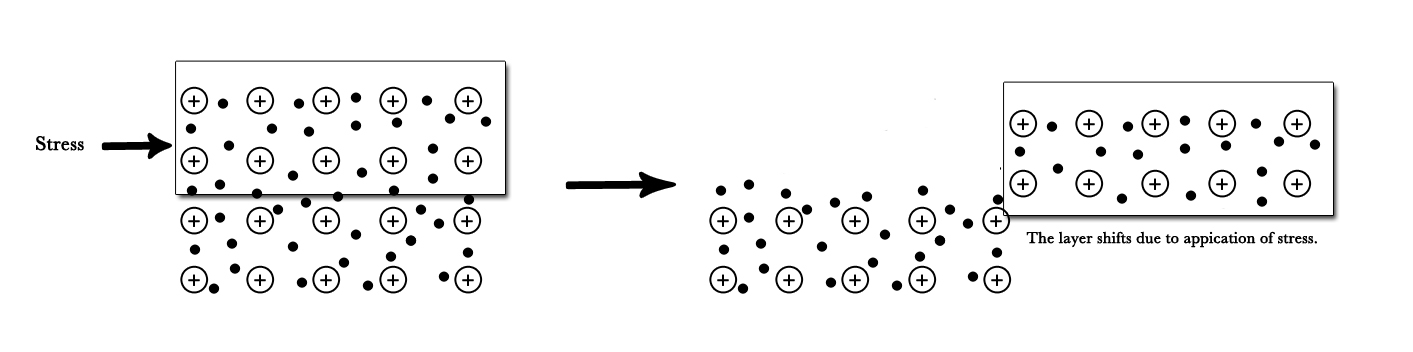
Malleability and Ductility – The metals are strong and are not brittle. When stress is applied to a metal surface, the layers of metal ions glide over each other. Thus, metals can be beaten into sheets, or drawn into wires. This explains why metals are malleable and ductile.
So now we know why we can easily make aluminum foils and gold coins! Why we can make beautiful ornaments using gold and silver, and why we can use copper wires to supply electricity to our homes!


Though this “electron-gas” model gave an adequate qualitative rationale for the metallic properties, it could not correctly predict the heat capacity and the temperature dependence of the electrical conductivity of metals.
The band theory, which was developed later, provided a better explanation of bonding in metals. We will study this theory in the next post. Till then,
Be a perpetual student of life and keep learning…
Good day!
References and Further Reading –
1.http://www.che.uc.edu/jensen/w.%20b.%20jensen/reprints/156.%20Metallic%20Bond.pdf
Image Source –
- http://clipart-library.com/gold-cliparts.html
- https://pngtree.com/freepng/copper-wire_1833738.html
- https://www.flickr.com/photos/maypldigitalart/28853776911
- https://pngtree.com/freepng/chow-tai-fook-platinum-fashion-rings_2080009.html
- https://en.wikipedia.org/wiki/Paul_Drude
- https://www.indiamart.com/proddetail/aluminium-foil-paper-15171454597.html
One comment