As discussed in the previous post, the free electron gas model was not infallible. Almost 30 years after the electron gas model was proposed, quantum chemistry changed the perspective of looking at bonding and thus a new approach to metallic bonding was stated.

Felix Bloch graduated in Zurich, Switzerland, and later went to Werner Heisenberg for his doctorate research. In 1928, he started applying quantum mechanical theories to solids. Until then these theories were studied with respect to gases only and this was the first attempt to apply them to the solids. He applied Schrödinger’s equation to solids and got a set of solutions. This study was a precursor of the band theory developed later.
The Band Theory Of Solids
The band theory of solids was developed by Walter Hitler and Fritz London.
In the 20th century, Quantum Mechanics started stirring up the scientific world. The quantum theory was deployed to explain many unaccountable scientific observations. Walter Hitler and Fritz London did the same. They applied the quantum theory of solids – the Molecular Orbital Theory (MOT ) – to the Avogadro number (6.023 ×1023) of atoms. When the linear combination of atomic orbitals (LCAO) approach is applied to such a large no of atomic orbitals(AOs) we get bands of molecular orbitals.
We have already studied in post #81, that two AOs form two MOs –
i)one lower energy bonding molecular orbital(BMO) σ and
ii) another higher energy antibonding molecular orbital(ABMO), σ*.
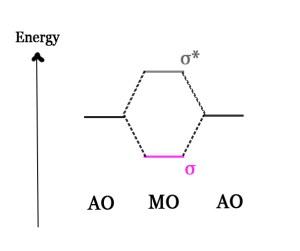
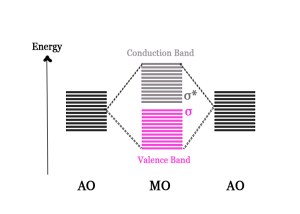
When this same approach is applied to an ensemble of atomic orbitals, we get very closely spaced BMOs and ABMOs, which appear like a band from a distance. The energy gap between them is very low, and so the levels appear to be in a continuum.
If N atomic orbitals combine to form N molecular orbitals, then N/2 will be bonding molecular orbitals and N/2 will be anti-bonding.
Two bands will be formed –
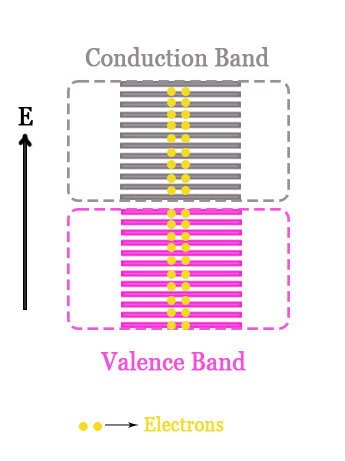
- Bonding Molecular Orbitals Band → This is called the BONDING/VALENCE BAND.
- Anti-bonding Molecular Orbitals Band → This is called the CONDUCTION BAND.
The energy gap between the valence and the conduction band is called BAND GAP/FORBIDDEN GAP/FORBIDDEN BAND.
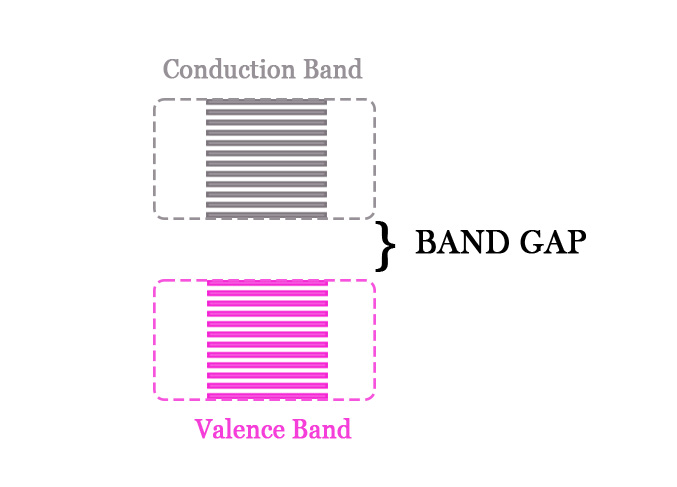
This band gap determines whether a material is a conductor, semi-conductor, or an insulator. If the band gap is too high, the material is an insulator. This is because the electrons in the valence band cannot easily move up to the conduction band. In semiconductors, this gap is a little less. In metals, however, there is no band gap. The valence and the conduction band are very close to each other and sometimes overlap too. With the application of a little potential difference, the electrons from the valence band can easily go to the conduction band and roam there. This is why metals are good conductors of electricity.

If there is only one electron in the valence shell of the atom, then the band will be half-filled. Electrons from two atoms will pair up in the valence band. The ABMOs/ conduction band will remain vacant. In this case, the energy gap between the highest occupied BMO and lowest unoccupied ABMO is so small, that with a little potential difference, electrons can be excited from the valence band to the vacant conduction band.
e.g. – Copper – [Ar] 3d10 4s1
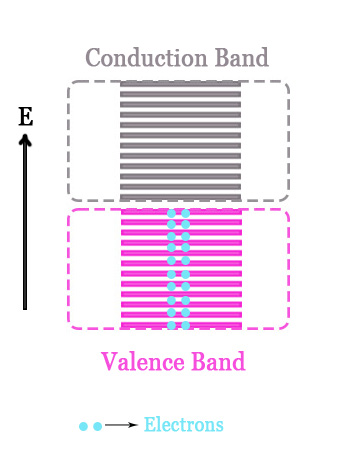
If there are two electrons in the valence shell of the atom, then the band will be fully filled i.e. all the electrons will pair up and fill all the orbitals. In this case, the ABMOs will NOT remain vacant.
e.g. -Manganese [Ar] 3d5 4s2
In this case, however, there are vacant p-orbitals (4p in the case of Mn) that form the p- band. The lower end of this vacant p-band lies a little lower in energy than the topmost end of the s-band (as shown in the figure below). Thus, electrons can be promoted to this p-band easily with the application of little potential difference. Thus, the band theory of solids helps explain why metals are good conductors of electricity.
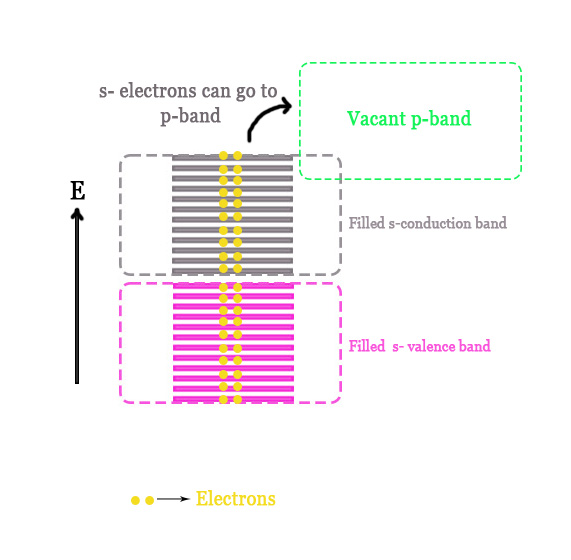
Thus, the band theory was successfully able to explain the conductivity in metals.
Metals have a shiny appearance. This is called luster. This theory could also give a plausible explanation for this luster. When light is shone upon a metal, the energy of this light is sufficient to excite the electrons to the vacant band in the metal structure. When these excited electrons cascade down, they emit light. This appears as a shine on the metal.
After discussing primary bonding in detail, we shall now move on to secondary bonding in our next post. Till then,
Be a perpetual student of life and keep learning…
Good day!
Image source –
1.https://en.wikipedia.org/wiki/Felix_Bloch
2.https://es.wikipedia.org/wiki/Walter_Heitler
3.https://phy.duke.edu/about/history/historical-faculty/fritz-london
References and Further Reading –
1.https://www.doitpoms.ac.uk/tlplib/semiconductors/energy_band_intro.php