We have already seen in post # 54, that the main difference between primary and secondary bonding is that in secondary bonding, there is NO SHARING OR TRANSFER OF ELECTRONS.
|
Primary bonding |
Secondary bonding |
|
sharing or transfer of electrons. |
NO sharing or transfer of electrons. |
| A strong force of attraction | A weak force of attraction |
| Short-range | Long-range |
|
e.g.- Ionic,Covalent ,Co-ordinate covalent,Metallic bonds |
e.g.- Hydrogen bonds, vander Waal’s forces, dipole interactions. |
From this post onwards we will discuss secondary bonding in detail.
SECONDARY BONDING
Secondary bonding arises in covalently bonded molecules due to the attraction of electric dipoles in atoms/molecules. This bonding is used to describe aggregation in molecules.
As mentioned in post # 54 there are three types of secondary bonds –
S1. Hydrogen bonds
S2. Vander Waals bonds / London dispersion force
S3. Dipole- Dipole interactions.
We shall begin by discussing the dipole interactions first, Vander Waals bonds second, and then the hydrogen bonding at last.
S3.DIPOLE-DIPOLE INTERACTIONS
This kind of secondary bonding is seen in polar molecules on account of asymmetric charge distribution. In polar molecules, the more electronegative atom pulls the shared electron cloud towards itself and thus it has a partial negative charge(-δ). A partial positive charge is developed on the other atom. These partial charges are permanent dipoles, which are responsible for the aggregation of such molecules. The positive end of one molecule gets attracted to the negative end of the other molecule and vice versa. This is the secondary bond – an intermolecular bond.
(Intermolecular ⇒ Between the molecules
Intramolecular ⇒ within the molecule).
The aggregate can be shown as follows –
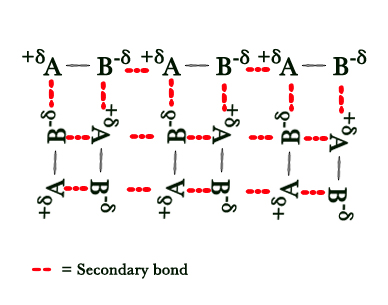
In the above figure – A⇒Electropositive atom & B ⇒ Electronegative atom.
The electron pair shared between A and B is attracted to the B atom more(as it is electronegative). Thus, B has a partial negative charge on it. As the electrons are more attracted towards B, A develops a partial positive charge. As seen above, the A of one molecule gets attracted to B of another molecule. This is the secondary bonding. (All the positive and negative ends are attracted towards each other and this dipole interaction is shown by a red dotted line).
e.g. HCl, CO, HF molecules.
The more the dipole moment (µ), the more is the interaction i.e these two quantities are directly proportional to each other. The energy of this interaction is directly proportional to the fourth power of dipole moment as shown below. It is inversely proportional to the distance (r) and temperature (T). At higher temperatures, the thermal energy acts as the disruptive force, thus decreasing the strength of dipole interactions.

Both ICl and Br2 have the same number of atoms and approximately the same molecular weight, but ICl is a solid whereas Br2 is a liquid at 0oC. Why?
Br2 is a homonuclear molecule and so it is not polar. Thus, there are no dipole interactions. However, in the ICl molecule, the electronegativities of iodine and chlorine atoms are significantly different and thus it is a polar molecule. Being a polar molecule, there are dipole interactions(cohesive force), which bring the adjacent molecules closer and at 0oC there are no disruptive forces (thermal energy ) too. Thus ICl exists in the liquid state.
In the next post, we shall discuss the Vander Waals bonds. Till then,
Be a perpetual student of life and keep learning …
Good day!
References and Further Reading –
1.https://www.chem.purdue.edu/gchelp/liquids/dipdip.html
2.https://www.youtube.com/watch?v=2Q_fna3TTbs&t=128s